The C-terminal heme regulatory motifs of heme oxygenase-2 are redox-regulated heme binding sites
- PMID: 25853617
- PMCID: PMC4478078
- DOI: 10.1021/acs.biochem.5b00266
The C-terminal heme regulatory motifs of heme oxygenase-2 are redox-regulated heme binding sites
Abstract
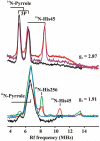
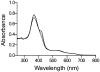
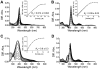
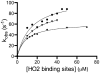
Heme oxygenase-2 (HO2), an enzyme that catalyzes the conversion of heme to biliverdin, contains three heme regulatory motifs (HRMs) centered at Cys127, Cys265, and Cys282. Previous studies using the soluble form of human HO2 spanning residues 1-288 (HO2sol) have shown that a disulfide bond forms between Cys265 and Cys282 and that, in this oxidized state, heme binds to the catalytic site of HO2sol via His45. However, various mutational and spectroscopic studies have confirmed the involvement of cysteine in Fe(3+)-heme binding upon reduction of the disulfide bond. In an effort to understand how the HRMs are involved in binding of heme to disulfide-reduced HO2sol, in the work described here, we further investigated the properties of Fe(3+)-heme bound to HO2. Specifically, we investigated binding of Fe(3+)-heme to a truncated form of soluble HO2 (residues 213-288; HO2tail) that spans the C-terminal HRMs of HO2 but lacks the catalytic core. We found that HO2tail in the disulfide-reduced state binds Fe(3+)-heme and accounts for the spectral features observed upon binding of heme to the disulfide-reduced form of HO2sol that cannot be attributed to heme binding at the catalytic site. Further analysis revealed that while HO2sol binds one Fe(3+)-heme per monomer of protein under oxidizing conditions, disulfide-reduced HO2sol binds slightly more than two. Both Cys265 and Cys282 were identified as Fe(3+)-heme ligands, and His256 also acts as a ligand to the Cys265-ligated heme. Additionally, Fe(3+)-heme binds with a much weaker affinity to Cys282 than to Cys265, which has an affinity much weaker than that of the His45 binding site in the catalytic core. In summary, disulfide-reduced HO2 has multiple binding sites with varying affinities for Fe(3+)-heme.
Figures




Similar articles
-
Comparison of the Mechanisms of Heme Hydroxylation by Heme Oxygenases-1 and -2: Kinetic and Cryoreduction Studies.Biochemistry. 2016 Jan 12;55(1):62-8. doi: 10.1021/acs.biochem.5b00943. Epub 2015 Dec 23. Biochemistry. 2016. PMID: 26652036 Free PMC article.
-
Spectroscopic studies reveal that the heme regulatory motifs of heme oxygenase-2 are dynamically disordered and exhibit redox-dependent interaction with heme.Biochemistry. 2015 May 5;54(17):2693-708. doi: 10.1021/bi501489r. Epub 2015 Apr 22. Biochemistry. 2015. PMID: 25849895 Free PMC article.
-
The heme-regulatory motifs of heme oxygenase-2 contribute to the transfer of heme to the catalytic site for degradation.J Biol Chem. 2020 Apr 17;295(16):5177-5191. doi: 10.1074/jbc.RA120.012803. Epub 2020 Mar 9. J Biol Chem. 2020. PMID: 32152224 Free PMC article.
-
Redox Regulation of Heme Oxygenase-2 and the Transcription Factor, Rev-Erb, Through Heme Regulatory Motifs.Antioxid Redox Signal. 2018 Dec 20;29(18):1841-1857. doi: 10.1089/ars.2017.7368. Epub 2017 Nov 14. Antioxid Redox Signal. 2018. PMID: 28990415 Free PMC article. Review.
-
Structure and catalytic mechanism of heme oxygenase.Nat Prod Rep. 2007 Jun;24(3):553-70. doi: 10.1039/b604180a. Epub 2007 Mar 8. Nat Prod Rep. 2007. PMID: 17534530 Review. No abstract available.
Cited by
-
Heme oxygenase-2 is post-translationally regulated by heme occupancy in the catalytic site.J Biol Chem. 2020 Dec 11;295(50):17227-17240. doi: 10.1074/jbc.RA120.014919. Epub 2020 Oct 13. J Biol Chem. 2020. PMID: 33051205 Free PMC article.
-
Tight binding of heme to Staphylococcus aureus IsdG and IsdI precludes design of a competitive inhibitor.Metallomics. 2017 May 24;9(5):556-563. doi: 10.1039/c7mt00035a. Metallomics. 2017. PMID: 28401968 Free PMC article.
-
Gasotransmitters in pregnancy: from conception to uterine involution.Biol Reprod. 2019 Jul 1;101(1):4-25. doi: 10.1093/biolre/ioz038. Biol Reprod. 2019. PMID: 30848786 Free PMC article. Review.
-
An Extended C-Terminus, the Possible Culprit for Differential Regulation of 5-Aminolevulinate Synthase Isoforms.Front Mol Biosci. 2022 Jul 14;9:920668. doi: 10.3389/fmolb.2022.920668. eCollection 2022. Front Mol Biosci. 2022. PMID: 35911972 Free PMC article. Review.
-
Heme Oxygenases in Cardiovascular Health and Disease.Physiol Rev. 2016 Oct;96(4):1449-508. doi: 10.1152/physrev.00003.2016. Physiol Rev. 2016. PMID: 27604527 Free PMC article. Review.
References
-
- Maines MD. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. - PubMed
-
- Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase: Only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986;261:411–419. - PubMed
-
- McCoubrey WK, Maines MD. Domains of rat heme oxygenase-2: The amino terminus and histidine-151 are required for heme oxidation. Arch. Biochem. Biophys. 1993;302:402–408. - PubMed
-
- Schuller DJ, Wilks A, de Montellano PRO, Poulos TL. Crystal structure of human heme oxygenase-l. Nat. Struct. Biol. 1999;6:860–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

