Marked seasonality and high spatial variability of protist communities in shallow freshwater systems
- PMID: 25853803
- PMCID: PMC4542043
- DOI: 10.1038/ismej.2015.6
Marked seasonality and high spatial variability of protist communities in shallow freshwater systems
Abstract
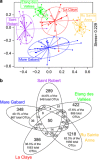
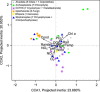
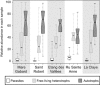
Small eukaryotes have key roles in aquatic ecosystems, influencing their local environment, global biogeochemical cycles and climate. Their impact depends on community structure, which varies along time. However, very few studies take into account temporal variation. This is especially true for small, shallow freshwater systems, which remain largely understudied despite their wide variety, global surface and intense microbial activity. We have monthly followed changes in the community structure of small microbial eukaryotes (0.2-5 μm cell diameter) for 2 years in four ponds and one brook located in North-Western France based on massive 18S rDNA amplicon 454 pyrosequencing. We detected a total of 3742 stringently defined operational taxonomic units (OTUs) encompassing all recognized eukaryotic supergroups and lineages of uncertain affiliation. Although geographically close, protist communities in the five ecosystems were contrasting, with very few shared OTUs, suggesting that environmental selection mainly drives community structure. The temporal dynamics of different high-rank taxa appeared complex and rapid at monthly scales. Despite this, a clear and reproducible seasonality was observed. As expected, low-abundance OTUs dominated the community. Although some of them appeared sporadically or remained at low frequencies during the survey, others occasionally reached relatively high abundances, sometimes recurrently. This shows that at least a fraction of low-abundance eukaryotes constitutes a seed bank. The annual proportion of primary producers, free-living heterotrophs and parasites appeared remarkably constant among the different ecosystems, suggesting underlying trends of ecosystem carrying capacity for these functional groups.
Figures



References
-
- Anderson MJ. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol 26: 32–46.
-
- Bamforth SS. (1958). ecological studies on the planktonic protozoa of a small artificial pond. Limnol Oceanogr 3: 398–412.
-
- Bolker B, Bonekakke L, Gentleman R, Liaw WHA, Lumley T, Maechler M et al. (2012), gplots: Various R programming tools for plotting data. R package version 2.11.0. Available at: http://cran.r-project.org/package=gplots.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

