Nitric oxide sustains IL-1β expression in human dendritic cells enhancing their capacity to induce IL-17-producing T-cells
- PMID: 25853810
- PMCID: PMC4390375
- DOI: 10.1371/journal.pone.0120134
Nitric oxide sustains IL-1β expression in human dendritic cells enhancing their capacity to induce IL-17-producing T-cells
Abstract
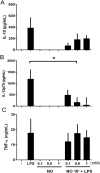
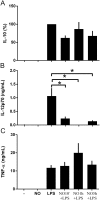
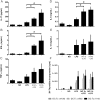
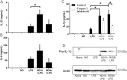
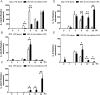
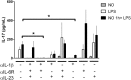
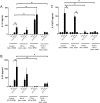
The role played by lung dendritic cells (DCs) which are influenced by external antigens and by their redox state in controlling inflammation is unclear. We studied the role played by nitric oxide (NO) in DC maturation and function. Human DCs were stimulated with a long-acting NO donor, DPTA NONOate, prior to exposure to lipopolysaccharide (LPS). Dose-and time-dependent experiments were performed with DCs with the aim of measuring the release and gene expression of inflammatory cytokines capable of modifying T-cell differentiation, towardsTh1, Th2 and Th17 cells. NO changed the pattern of cytokine release by LPS-matured DCs, dependent on the concentration of NO, as well as on the timing of its addition to the cells during maturation. Addition of NO before LPS-induced maturation strongly inhibited the release of IL-12, while increasing the expression and release of IL-23, IL-1β and IL-6, which are all involved in Th17 polarization. Indeed, DCs treated with NO efficiently induced the release of IL-17 by T-cells through IL-1β. Our work highlights the important role that NO may play in sustaining inflammation during an infection through the preferential differentiation of the Th17 lineage.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

