Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia
- PMID: 25853898
- PMCID: PMC4502662
- DOI: 10.1080/15548627.2015.1034414
Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia
Abstract
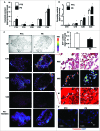
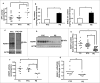
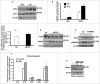
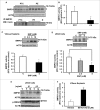
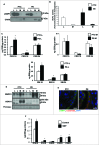
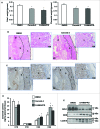
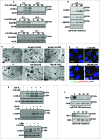
Bioactive sphingolipids including ceramides are involved in a variety of pathophysiological processes by regulating cell death and survival. The objective of the current study was to examine ceramide metabolism in preeclampsia, a serious disorder of pregnancy characterized by oxidative stress, and increased trophoblast cell death and autophagy. Maternal circulating and placental ceramide levels quantified by tandem mass spectrometry were elevated in pregnancies complicated by preeclampsia. Placental ceramides were elevated due to greater de novo synthesis via high serine palmitoyltransferase activity and reduced lysosomal breakdown via diminished ASAH1 expression caused by TGFB3-induced E2F4 transcriptional repression. SMPD1 activity was reduced; hence, sphingomyelin degradation by SMPD1 did not contribute to elevated ceramide levels in preeclampsia. Oxidative stress triggered similar changes in ceramide levels and acid hydrolase expression in villous explants and trophoblast cells. MALDI-imaging mass spectrometry localized the ceramide increases to the trophophoblast layers and syncytial knots of placentae from pregnancies complicated by preeclampsia. ASAH1 inhibition or ceramide treatment induced autophagy in human trophoblast cells via a shift of the BOK-MCL1 rheostat toward prodeath BOK. Pharmacological inhibition of ASAH1 activity in pregnant mice resulted in increased placental ceramide content, abnormal placentation, reduced fetal growth, and increased autophagy via a similar shift in the BOK-MCL1 system. Our results reveal that oxidative stress-induced reduction of lysosomal hydrolase activities in combination with elevated de novo synthesis leads to ceramide overload, resulting in increased trophoblast cell autophagy, and typifies preeclampsia as a sphingolipid storage disorder.
Keywords: 2-OE, 2-oleoylethanolamine; 3-KDS, 3-keto dihydrosphingosine; 3-MA, 3-methyladenine; ACTB, actin β; ASAH1, N-acylsphingosine amidohydrolase (acid ceramidase) 1; BECN1, Beclin 1, autophagy related; BOK; BOK, BCL2-related ovarian killer; BafA1, bafilomycin A1; CANX, calnexin; CASP3 (caspase 3, apoptosis-related cysteine peptidase); CERs, ceramides; CT, cytotrophoblast cells; D-NMAPPD, N-[(1R,2R)-2-hydroxyl-1-(hydroxyL-methyl)-2-(4-nitrophenyl) ethyl]-tetradecanamide; DHCer, dihydro-ceramide; E2F4, E2F transcription factor 4, p107/p130-binding; HIF1A, hypoxia inducible factor 1, α, subunit (basic helix-loop-helix transcription factor); LAMP1, lysosomal-associated membrane protein 1; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LC3B-II, cleaved and lipidated form of microtubule-associated protein 1 light chain 3 β (MAP1LC3B/LC3B); MALDI-MS, matrix-assisted laser desorption/ionization-mass spectrometry; MCL1; MCL1, myeloid cell leukemia 1; PE, preeclampsia; PTC, preterm control; S1P, sphingosine-1-phosphate; SM, sphingomyelin; SMPD1, sphingomyelin phosphodiesterase 1, acid lysosomal (acid sphingomyelinase); SNP, sodium nitroprusside (III); SPH, sphingosine; SPT, serine palmitoyltransferase; SQSTM1/p62, sequestosome 1; ST, syncytium/syncytiotrophoblast cells; Sa, sphinganine; TC, term control; TGFB, transforming growth factor β; autophagy; oxidative stress; placenta; preeclampsia; siRNA, small-interfering ribonucleic acid; sphingolipid metabolism.
Figures
References
-
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008; 9:139-50; PMID:18216770; http://dx.doi.org/ 10.1038/nrm2329 - DOI - PubMed
-
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science 1996; 274:1855-9; PMID:8943189; http://dx.doi.org/ 10.1126/science.274.5294.1855 - DOI - PubMed
-
- Ferlinz K, Kopal G, Bernardo K, Linke T, Bar J, Breiden B, Neumann U, Lang F, Schuchman EH, Sandhoff K. Human acid ceramidase: processing, glycosylation, and lysosomal targeting. J Biol Chem 2001; 276:35352-60; PMID:11451951; http://dx.doi.org/ 10.1074/jbc.M103066200 - DOI - PubMed
-
- Ferlinz K, Hurwitz R, Moczall H, Lansmann S, Schuchman EH, Sandhoff K. Functional characterization of the N-glycosylation sites of human acid sphingomyelinase by site-directed mutagenesis. Eur J Biochem 1997; 243:511-7; PMID:9030779; http://dx.doi.org/ 10.1111/j.1432-1033.1997.511_1a.x - DOI - PubMed
-
- Jenkins RW, Idkowiak-Baldys J, Simbari F, Canals D, Roddy P, Riner CD, Clarke JC, Hannun YA. A novel mechanism of lysosomal acid sphingomyelinase maturation: requirement for carboxyl-terminal proteolytic processing. J Biol Chem 2011; 286:3777-88; PMID:21098024; http://dx.doi.org/ 10.1074/jbc.M110.155234 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
