Expression of the ALS-causing variant hSOD1(G93A) leads to an impaired integrity and altered regulation of claudin-5 expression in an in vitro blood-spinal cord barrier model
- PMID: 25853911
- PMCID: PMC4640277
- DOI: 10.1038/jcbfm.2015.57
Expression of the ALS-causing variant hSOD1(G93A) leads to an impaired integrity and altered regulation of claudin-5 expression in an in vitro blood-spinal cord barrier model
Abstract
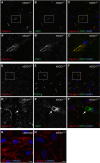
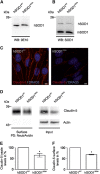
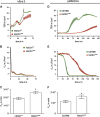
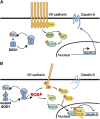
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder characterized by progressive paralysis due to the loss of primary and secondary motor neurons. Mutations in the Cu/Zn-superoxide dismutase (SOD1) gene are associated with familial ALS and to date numerous hypotheses for ALS pathology exist including impairment of the blood-spinal cord barrier. In transgenic mice carrying mutated SOD1 genes, a disrupted blood-spinal cord barrier as well as decreased levels of tight junction (TJ) proteins ZO-1, occludin, and claudin-5 were detected. Here, we examined TJ protein levels and barrier function of primary blood-spinal cord barrier endothelial cells of presymptomatic hSOD1(G93A) mice and bEnd.3 cells stably expressing hSOD1(G93A). In both cellular systems, we observed reduced claudin-5 levels and a decreased transendothelial resistance (TER) as well as an increased apparent permeability. Analysis of the β-catenin/AKT/forkhead box protein O1 (FoxO1) pathway and the FoxO1-regulated activity of the claudin-5 promoter revealed a repression of the claudin-5 gene expression in hSOD1(G93A) cells, which was depended on the phosphorylation status of FoxO1. These results strongly indicate that mutated SOD1 affects the expression and localization of TJ proteins leading to impaired integrity and breakdown of the blood-spinal cord barrier.
Figures


Similar articles
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
Mutant superoxide dismutase aggregates from human spinal cord transmit amyotrophic lateral sclerosis.Acta Neuropathol. 2018 Dec;136(6):939-953. doi: 10.1007/s00401-018-1915-y. Epub 2018 Oct 3. Acta Neuropathol. 2018. PMID: 30284034 Free PMC article.
-
Nuclear localization of human SOD1 and mutant SOD1-specific disruption of survival motor neuron protein complex in transgenic amyotrophic lateral sclerosis mice.J Neuropathol Exp Neurol. 2012 Feb;71(2):162-77. doi: 10.1097/NEN.0b013e318244b635. J Neuropathol Exp Neurol. 2012. PMID: 22249462 Free PMC article.
-
Barrier function in the peripheral and central nervous system-a review.Pflugers Arch. 2017 Jan;469(1):123-134. doi: 10.1007/s00424-016-1920-8. Epub 2016 Dec 12. Pflugers Arch. 2017. PMID: 27957611 Review.
-
Claudin-5: gatekeeper of neurological function.Fluids Barriers CNS. 2019 Jan 29;16(1):3. doi: 10.1186/s12987-019-0123-z. Fluids Barriers CNS. 2019. PMID: 30691500 Free PMC article. Review.
Cited by
-
Phenotypic characteristics of human bone marrow-derived endothelial progenitor cells in vitro support cell effectiveness for repair of the blood-spinal cord barrier in ALS.Brain Res. 2019 Dec 1;1724:146428. doi: 10.1016/j.brainres.2019.146428. Epub 2019 Sep 4. Brain Res. 2019. PMID: 31493389 Free PMC article.
-
Impaired tissue barriers as potential therapeutic targets for Parkinson's disease and amyotrophic lateral sclerosis.Metab Brain Dis. 2018 Aug;33(4):1031-1043. doi: 10.1007/s11011-018-0239-x. Epub 2018 Apr 22. Metab Brain Dis. 2018. PMID: 29681010 Review.
-
Endothelial LRP1 - A Potential Target for the Treatment of Alzheimer's Disease : Theme: Drug Discovery, Development and Delivery in Alzheimer's Disease Guest Editor: Davide Brambilla.Pharm Res. 2017 Dec;34(12):2637-2651. doi: 10.1007/s11095-017-2267-3. Epub 2017 Sep 25. Pharm Res. 2017. PMID: 28948494 Review.
-
Endothelial Dysfunctions in Blood-Brain Barrier Breakdown in Alzheimer's Disease: From Mechanisms to Potential Therapies.CNS Neurosci Ther. 2024 Nov;30(11):e70079. doi: 10.1111/cns.70079. CNS Neurosci Ther. 2024. PMID: 39548663 Free PMC article. Review.
-
Barriers in the Nervous System: Challenges and Opportunities for Novel Biomarkers in Amyotrophic Lateral Sclerosis.Cells. 2025 Jun 5;14(11):848. doi: 10.3390/cells14110848. Cells. 2025. PMID: 40498024 Free PMC article. Review.
References
-
- 1Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 2013; 14: 248–264. - PubMed
-
- 2Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993; 362: 59–62. - PubMed
-
- 3Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995; 64: 97–112. - PubMed
-
- 4Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 1994; 264: 1772–1775. - PubMed
-
- 5Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W. The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci 2010; 31: 2247–2265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

