Processing of visually evoked innate fear by a non-canonical thalamic pathway
- PMID: 25854147
- PMCID: PMC4403372
- DOI: 10.1038/ncomms7756
Processing of visually evoked innate fear by a non-canonical thalamic pathway
Erratum in
-
Corrigendum: Processing of visually evoked innate fear by a non-canonical thalamic pathway.Nat Commun. 2015 Aug 21;6:8228. doi: 10.1038/ncomms9228. Nat Commun. 2015. PMID: 26293832 Free PMC article. No abstract available.
Abstract
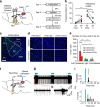
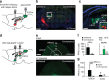
The ability of animals to respond to life-threatening stimuli is essential for survival. Although vision provides one of the major sensory inputs for detecting threats across animal species, the circuitry underlying defensive responses to visual stimuli remains poorly defined. Here, we investigate the circuitry underlying innate defensive behaviours elicited by predator-like visual stimuli in mice. Our results demonstrate that neurons in the superior colliculus (SC) are essential for a variety of acute and persistent defensive responses to overhead looming stimuli. Optogenetic mapping revealed that SC projections to the lateral posterior nucleus (LP) of the thalamus, a non-canonical polymodal sensory relay, are sufficient to mimic visually evoked fear responses. In vivo electrophysiology experiments identified a di-synaptic circuit from SC through LP to the lateral amygdale (Amg), and lesions of the Amg blocked the full range of visually evoked defensive responses. Our results reveal a novel collicular-thalamic-Amg circuit important for innate defensive responses to visual threats.
Figures




References
-
- Blanchard R. J. & Blanchard D. C. Defensive reactions in the albino rat. Learn Motiv. 2, 351–362 (1971) .
-
- Wiener S. G. & Levine S. Behavioural and physiological responses of mother and infant squirrel monkeys to fearful stimuli. Dev. Psychobiol. 25, 127–136 (1992) . - PubMed
-
- Sewards T. V. & Sewards M. A. Innate visual object recognition in vertebrates: some proposed pathways and mechanisms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 132, 861–891 (2002) . - PubMed
-
- Vagnoni E., Lourenco S. F. & Longo M. R. Threat modulates perception of looming visual stimuli. Curr. Biol. 22, R826–R827 (2012) . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

