A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: a Children's Oncology Group study†
- PMID: 25854526
- PMCID: PMC4490875
- DOI: 10.1093/neuonc/nov057
A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: a Children's Oncology Group study†
Abstract
Background: This study was initiated to test the feasibility and toxicity of a regimen that alternates the administration of weekly carboplatin and vincristine with temozolomide in the management of children with progressive and/or symptomatic low-grade glioma.
Methods: Eligible children received a 10-week induction regimen followed by six 10-week cycles of maintenance chemotherapy. Feasibility was evaluated with short-term and long-term endpoints. Short-term feasibility was evaluated by the ability to complete induction and 1 maintenance cycle in 24 weeks without >25% reduction in either carboplatin or temozolomide. Long-term feasibility was evaluated by the ability to administer induction and 4 maintenance cycles within 60 weeks without >25% reduction in either carboplatin or temozolomide. Efficacy was assessed by response to initial chemotherapy and 5-year event-free survival. Initial pathology was reviewed centrally.
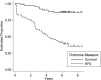
Results: Sixty-six patients were enrolled on the study. It was feasible to deliver the regimen, and toxicity was acceptable. The only significant toxicities were hematologic. Both the short-term and long-term feasibility endpoints were met. The short-term feasibility success rate was 87% (95% CI: 77%-96%) and the long-term feasibility success rate was 79% (95% CI: 68%-90%). The 5-year event-free survival was 46% (95% CI: 33%-58%) and the 5-year survival was 87% (95% CI: 75%-93%).
Conclusion: It was feasible to deliver the combination of weekly carboplatin and vincristine alternating with temozolomide to children with progressive/symptomatic low-grade glioma with acceptable toxicities. This combination appears to be effective in delaying progression. Further trials are needed to establish the relative efficacy of this regimen compared with other regimens in use.
Keywords: chemotherapy; low-grade glioma; temozolomide.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Pollack IF. Brain tumors in children. N Engl J Med. 1994;331(22):1500–1507. - PubMed
-
- Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15(8):2792–2799. - PubMed
-
- Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11(5):850–856. - PubMed
-
- Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. - PubMed
-
- Petronio J, Edwards MS, Prados M, et al. Management of chiasmal and hypothalamic gliomas of infancy and childhood with chemotherapy. J Neurosurg. 1991;74(5):701–708. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous