Perspectives on genetic and genomic technologies in an academic medical center: the duke experience
- PMID: 25854543
- PMCID: PMC4493486
- DOI: 10.3390/jpm5020067
Perspectives on genetic and genomic technologies in an academic medical center: the duke experience
Abstract
In this age of personalized medicine, genetic and genomic testing is expected to become instrumental in health care delivery, but little is known about its actual implementation in clinical practice.
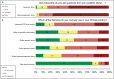
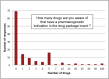
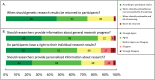
Methods: We surveyed Duke faculty and healthcare providers to examine the extent of genetic and genomic testing adoption. We assessed providers' use of genetic and genomic testing options and indications in clinical practice, providers' awareness of pharmacogenetic applications, and providers' opinions on returning research-generated genetic test results to participants. Most clinician respondents currently use family history routinely in their clinical practice, but only 18 percent of clinicians use pharmacogenetics. Only two respondents correctly identified the number of drug package inserts with pharmacogenetic indications. We also found strong support for the return of genetic research results to participants. Our results demonstrate that while Duke healthcare providers are enthusiastic about genomic technologies, use of genomic tools outside of research has been limited. Respondents favor return of research-based genetic results to participants, but clinicians lack knowledge about pharmacogenetic applications. We identified challenges faced by this institution when implementing genetic and genomic testing into patient care that should inform a policy and education agenda to improve provider support and clinician-researcher partnerships.
Figures
Similar articles
-
Perceptions of Personalized Medicine in an Academic Health System: Educational Findings.J Contemp Med Educ. 2015;3(1):14-19. doi: 10.5455/jcme.20150408050414. J Contemp Med Educ. 2015. PMID: 26236542 Free PMC article.
-
Dental screening and referral of young children by pediatric primary care providers.Pediatrics. 2004 Nov;114(5):e642-52. doi: 10.1542/peds.2004-1269. Pediatrics. 2004. PMID: 15520094
-
Evidence-based pharmacogenetics: Is it possible?Int J Risk Saf Med. 2015;27 Suppl 1:S97-8. doi: 10.3233/JRS-150706. Int J Risk Saf Med. 2015. PMID: 26639733
-
Educational and Ethical Considerations for Genetic Test Implementation Within Health Care Systems.Netw Syst Med. 2020 May 26;3(1):58-66. doi: 10.1089/nsm.2019.0010. eCollection 2020. Netw Syst Med. 2020. PMID: 32676590 Free PMC article. Review.
-
The state of genomic health care and cancer. Are we going two steps forward and one step backward?Annu Rev Nurs Res. 2011;29:73-97. doi: 10.1891/0739-6686.29.73. Annu Rev Nurs Res. 2011. PMID: 22891499 Review.
Cited by
-
Perceptions of Personalized Medicine in an Academic Health System: Educational Findings.J Contemp Med Educ. 2015;3(1):14-19. doi: 10.5455/jcme.20150408050414. J Contemp Med Educ. 2015. PMID: 26236542 Free PMC article.
-
Are Graduate Medical Trainees Prepared for the Personalized Genomic Medicine Revolution? Trainee Perspectives at One Institution.J Pers Med. 2023 Jun 21;13(7):1025. doi: 10.3390/jpm13071025. J Pers Med. 2023. PMID: 37511638 Free PMC article.
-
Practical considerations for implementing genomic information resources. Experiences from eMERGE and CSER.Appl Clin Inform. 2016 Sep 21;7(3):870-82. doi: 10.4338/ACI-2016-04-RA-0060. Appl Clin Inform. 2016. PMID: 27652374 Free PMC article.
-
Hospital nursing leadership-led interventions increased genomic awareness and educational intent in Magnet settings.Nurs Outlook. 2018 May-Jun;66(3):244-253. doi: 10.1016/j.outlook.2017.10.010. Epub 2017 Nov 13. Nurs Outlook. 2018. PMID: 29544651 Free PMC article.
-
Beyond BRCA: A Pilot Program to Assess and Improve Knowledge of Pharmacogenomic Testing Among Advanced Practitioners in a Breast Cancer Treatment Setting.J Adv Pract Oncol. 2016 May-Jun;7(4):382-389. doi: 10.6004/jadpro.2016.7.4.2. Epub 2016 May 1. J Adv Pract Oncol. 2016. PMID: 29225997 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

