Spinocerebellar ataxia type 19/22 mutations alter heterocomplex Kv4.3 channel function and gating in a dominant manner
- PMID: 25854634
- PMCID: PMC4531139
- DOI: 10.1007/s00018-015-1894-2
Spinocerebellar ataxia type 19/22 mutations alter heterocomplex Kv4.3 channel function and gating in a dominant manner
Abstract
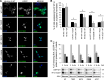
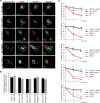
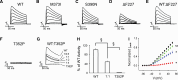
The dominantly inherited cerebellar ataxias are a heterogeneous group of neurodegenerative disorders caused by Purkinje cell loss in the cerebellum. Recently, we identified loss-of-function mutations in the KCND3 gene as the cause of spinocerebellar ataxia type 19/22 (SCA19/22), revealing a previously unknown role for the voltage-gated potassium channel, Kv4.3, in Purkinje cell survival. However, how mutant Kv4.3 affects wild-type Kv4.3 channel functioning remains unknown. We provide evidence that SCA19/22-mutant Kv4.3 exerts a dominant negative effect on the trafficking and surface expression of wild-type Kv4.3 in the absence of its regulatory subunit, KChIP2. Notably, this dominant negative effect can be rescued by the presence of KChIP2. We also found that all SCA19/22-mutant subunits either suppress wild-type Kv4.3 current amplitude or alter channel gating in a dominant manner. Our findings suggest that altered Kv4.3 channel localization and/or functioning resulting from SCA19/22 mutations may lead to Purkinje cell loss, neurodegeneration and ataxia.
Conflict of interest statement
The authors declare that they do not have any competing or financial interests.
Figures


References
-
- Lee YC, Durr A, Majczenko K, Huang YH, Liu YC, Lien CC, Tsai PC, Ichikawa Y, Goto J, Monin ML, Li JZ, Chung MY, Mundwiller E, Shakkottai V, Liu TT, Tesson C, Lu YC, Brice A, Tsuji S, Burmeister M, Stevanin G, Soong BW. Mutations in KCND3 cause spinocerebellar ataxia type 22. Ann Neurol. 2012;72:859–869. doi: 10.1002/ana.23701. - DOI - PMC - PubMed
-
- Duarri A, Jezierska J, Fokkens M, Meijer M, Schelhaas HJ, den Dunnen WF, van Dijk F, Verschuuren-Bemelmans C, Hageman G, van de Vlies P, Kusters B, van de Warrenburg BP, Kremer B, Wijmenga C, Sinke RJ, Swertz MA, Kampinga HH, Boddeke E, Verbeek DS. Mutations in potassium channel kcnd3 cause spinocerebellar ataxia type 19. Ann Neurol. 2012;72:870–880. doi: 10.1002/ana.23700. - DOI - PubMed
-
- Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–36454. doi: 10.1074/jbc.M306142200. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

