Marine extremophiles: a source of hydrolases for biotechnological applications
- PMID: 25854643
- PMCID: PMC4413194
- DOI: 10.3390/md13041925
Marine extremophiles: a source of hydrolases for biotechnological applications
Abstract
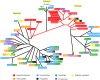
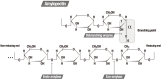
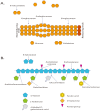
The marine environment covers almost three quarters of the planet and is where evolution took its first steps. Extremophile microorganisms are found in several extreme marine environments, such as hydrothermal vents, hot springs, salty lakes and deep-sea floors. The ability of these microorganisms to support extremes of temperature, salinity and pressure demonstrates their great potential for biotechnological processes. Hydrolases including amylases, cellulases, peptidases and lipases from hyperthermophiles, psychrophiles, halophiles and piezophiles have been investigated for these reasons. Extremozymes are adapted to work in harsh physical-chemical conditions and their use in various industrial applications such as the biofuel, pharmaceutical, fine chemicals and food industries has increased. The understanding of the specific factors that confer the ability to withstand extreme habitats on such enzymes has become a priority for their biotechnological use. The most studied marine extremophiles are prokaryotes and in this review, we present the most studied archaea and bacteria extremophiles and their hydrolases, and discuss their use for industrial applications.
Figures

References
-
- BCC Research . Global Markets for Enzymes in Industrial Applications. BCC Research; Wellesley, MA, USA: 2014. p. 146. BIO030H.
-
- Díaz-Tenaa E., Rodríguez-Ezquerroa A., Marcaidea L.N.L.L., Bustinduyb L.G., Sáenzb A.E. Use of Extremophiles Microorganisms for Metal Removal. Procedia Eng. 2013;63:67–74. doi: 10.1016/j.proeng.2013.08.197. - DOI
-
- Vermelho A.B., Noronha E.F., Filho E.X., Ferrara M.A., Bon E.P.S. Diversity and biotechnological applications of prokaryotic enzymes. In: Rosenberg E., DeeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes. Springer-Verlag Heidelberg; Berlin, Germany: 2013. pp. 213–240.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

