Probing the relationship between anti-Pneumocystis carinii activity and DNA binding of bisamidines by molecular dynamics simulations
- PMID: 25854757
- PMCID: PMC6272165
- DOI: 10.3390/molecules20045942
Probing the relationship between anti-Pneumocystis carinii activity and DNA binding of bisamidines by molecular dynamics simulations
Abstract
The anti-Pneumocystis carinii activity of 13 synthetic pentamidine analogs was analyzed. The experimental differences in melting points of DNA dodecamer 5'-(CGCGAATTCGCG)2-3' complexes (ΔTm), and in the biological activity measured using ATP bioluminescence assay (IC50) together with the theoretical free energy of DNA-ligand binding estimated by the proposed computational protocol, showed that the experimental activity of the tested pentamidines appeared to be due to the binding to the DNA minor groove with extended AT sequences. The effect of heteroatoms in the aliphatic linker, and the sulfonamide or methoxy substituents on the compound inducing changes in the interactions with the DNA minor groove was examined and was correlated with biological activity. In computational analysis, the explicit solvent approximation with the discrete water molecules was taken into account, and the role of water molecules in the DNA-ligand complexes was defined.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




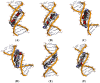





References
-
- Mathis A.M., Holman J.L., Sturk L.M., Ismail M.A., Boykin D.W., Tidwell R.R., Hall J.E. Acumulation and intracelular distribution of Antitrypasomal diamidine compounds DB75 and DB820 in African Trypanosomes. Antimicrob. Agents Chemother. 2006;50:2185–2191. doi: 10.1128/AAC.00192-06. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

