Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury
- PMID: 25855125
- PMCID: PMC4515210
- DOI: 10.1002/hep.27841
Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury
Abstract
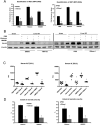
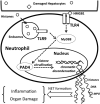
Innate immunity plays a crucial role in the response to sterile inflammation such as liver ischemia/reperfusion (I/R) injury. The initiation of liver I/R injury results in the release of damage-associated molecular patterns, which trigger an innate immune and inflammatory cascade through pattern recognition receptors. Neutrophils are recruited to the liver after I/R and contribute to organ damage and innate immune and inflammatory responses. Formation of neutrophil extracellular traps (NETs) has been recently found in response to various stimuli. However, the role of NETs during liver I/R injury remains unknown. We show that NETs form in the sinusoids of ischemic liver lobes in vivo. This was associated with increased NET markers, serum level of myeloperoxidase-DNA complexes, and tissue level of citrullinated-histone H3 compared to control mice. Treatment with peptidyl-arginine-deiminase 4 inhibitor or DNase I significantly protected hepatocytes and reduced inflammation after liver I/R as evidenced by inhibition of NET formation, indicating the pathophysiological role of NETs in liver I/R injury. In vitro, NETs increase hepatocyte death and induce Kupffer cells to release proinflammatory cytokines. Damage-associated molecular patterns, such as High Mobility Group Box 1 and histones, released by injured hepatocytes stimulate NET formation through Toll-like receptor (TLR4)- and TLR9-MyD88 signaling pathways. After neutrophil depletion in mice, the adoptive transfer of TLR4 knockout or TLR9 knockout neutrophils confers significant protection from liver I/R injury with a significant decrease in NET formation. In addition, we found inhibition of NET formation by the peptidyl-arginine-deiminase 4 inhibitor and that DNase I reduces High Mobility Group Box 1 and histone-mediated liver I/R injury.
Conclusion: Damage-associated molecular patterns released during liver I/R promote NET formation through the TLR signaling pathway. Development of NETs subsequently exacerbates organ damage and initiates inflammatory responses during liver I/R.
© 2015 by the American Association for the Study of Liver Diseases.
Figures






References
-
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. - PubMed
-
- Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677. - PubMed
-
- van Golen RF, Reiniers MJ, Olthof PB, van Gulik TM, Heger M. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. J Gastroenterol Hepatol. 2013;28:394–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
