Axonal amphoterin mRNA is regulated by translational control and enhances axon outgrowth
- PMID: 25855182
- PMCID: PMC4388927
- DOI: 10.1523/JNEUROSCI.3397-14.2015
Axonal amphoterin mRNA is regulated by translational control and enhances axon outgrowth
Abstract
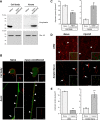
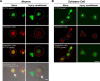
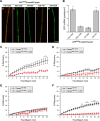
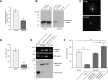
High mobility group (HMG) proteins concentrate in the nucleus, interacting with chromatin. Amphoterin is an HMG protein (HMGB1) that has been shown to have extranuclear functions and can be secreted from some cell types. Exogenous amphoterin can increase neurite growth, suggesting that the secreted protein may have growth promoting activities in neurons. Consistent with this, we show that depletion of amphoterin mRNA from cultured adult rat DRG neurons attenuates neurite outgrowth, pointing to autocrine or paracrine mechanisms for its growth-promoting effects. The mRNA encoding amphoterin localizes to axonal processes and we showed recently that its 3'-UTR is sufficient for axonal localization of heterologous transcripts (Donnelly et al., 2013). Here, we show that amphoterin mRNA is transported constitutively into axons of adult DRG neurons. A preconditioning nerve injury increases the levels of amphoterin protein in axons without a corresponding increase in amphoterin mRNA in the axons. A 60 nucleotide region of the amphoterin mRNA 3'-UTR is necessary and sufficient for its localization into axons of cultured sensory neurons. Amphoterin mRNA 3'-UTR is also sufficient for axonal localization in distal axons of DRG neurons in vivo. Overexpression of axonally targeted amphoterin mRNA increases axon outgrowth in cultured sensory neurons, but axon growth is not affected when the overexpressed mRNA is restricted to the cell body.
Keywords: HMGB1; axon regeneration; high mobility group protein; mRNA translation; mRNA transport.
Copyright © 2015 the authors 0270-6474/15/355693-14$15.00/0.
Figures


References
-
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
