Miro's N-terminal GTPase domain is required for transport of mitochondria into axons and dendrites
- PMID: 25855186
- PMCID: PMC4388930
- DOI: 10.1523/JNEUROSCI.1035-14.2015
Miro's N-terminal GTPase domain is required for transport of mitochondria into axons and dendrites
Abstract
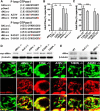
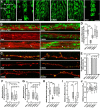
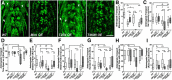
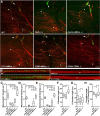
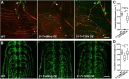
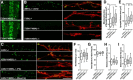
Mitochondria are dynamically transported in and out of neuronal processes to maintain neuronal excitability and synaptic function. In higher eukaryotes, the mitochondrial GTPase Miro binds Milton/TRAK adaptor proteins linking microtubule motors to mitochondria. Here we show that Drosophila Miro (dMiro), which has previously been shown to be required for kinesin-driven axonal transport, is also critically required for the dynein-driven distribution of mitochondria into dendrites. In addition, we used the loss-of-function mutations dMiroT25N and dMiroT460N to determine the significance of dMiro's N-terminal and C-terminal GTPase domains, respectively. Expression of dMiroT25N in the absence of endogenous dMiro caused premature lethality and arrested development at a pupal stage. dMiroT25N accumulated mitochondria in the soma of larval motor and sensory neurons, and prevented their kinesin-dependent and dynein-dependent distribution into axons and dendrites, respectively. dMiroT25N mutant mitochondria also were severely fragmented and exhibited reduced kinesin and dynein motility in axons. In contrast, dMiroT460N did not impair viability, mitochondrial size, or the distribution of mitochondria. However, dMiroT460N reduced dynein motility during retrograde mitochondrial transport in axons. Finally, we show that substitutions analogous to the constitutively active Ras-G12V mutation in dMiro's N-terminal and C-terminal GTPase domains cause neomorphic phenotypic effects that are likely unrelated to the normal function of each GTPase domain. Overall, our analysis indicates that dMiro's N-terminal GTPase domain is critically required for viability, mitochondrial size, and the distribution of mitochondria out of the neuronal soma regardless of the employed motor, likely by promoting the transition from a stationary to a motile state.
Keywords: Miro; mitochondria; transport.
Copyright © 2015 the authors 0270-6474/15/355754-18$15.00/0.
Figures





References
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
