Facilitation of corticostriatal transmission following pharmacological inhibition of striatal phosphodiesterase 10A: role of nitric oxide-soluble guanylyl cyclase-cGMP signaling pathways
- PMID: 25855188
- PMCID: PMC4388932
- DOI: 10.1523/JNEUROSCI.1238-14.2015
Facilitation of corticostriatal transmission following pharmacological inhibition of striatal phosphodiesterase 10A: role of nitric oxide-soluble guanylyl cyclase-cGMP signaling pathways
Abstract
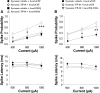
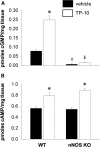
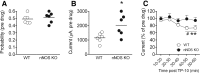
The striatum contains a rich variety of cyclic nucleotide phosphodiesterases (PDEs), which play a critical role in the regulation of cAMP and cGMP signaling. The dual-substrate enzyme PDE10A is the most highly expressed PDE in striatal medium-sized spiny neurons (MSNs) with low micromolar affinity for both cyclic nucleotides. Previously, we have shown that systemic and local administration of the selective PDE10A inhibitor TP-10 potently increased the responsiveness of MSNs to cortical stimulation. However, the signaling mechanisms underlying PDE10A inhibitor-induced changes in corticostriatal transmission are only partially understood. The current studies assessed the respective roles of cAMP and cGMP in the above effects using soluble guanylyl cyclase (sGC) or adenylate cyclase (AC) specific inhibitors. Cortically evoked spike activity was monitored in urethane-anesthetized rats using in vivo extracellular recordings performed proximal to a microdialysis probe during local infusion of vehicle, the selective sGC inhibitor ODQ, or the selective AC inhibitor SQ 22536. Systemic administration of TP-10 (3.2 mg/kg) robustly increased cortically evoked spike activity in a manner that was blocked following intrastriatal infusion of ODQ (50 μm). The effects of TP-10 on evoked activity were due to accumulation of cGMP, rather than cAMP, as the AC inhibitor SQ was without effect. Consistent with these observations, studies in neuronal NO synthase (nNOS) knock-out (KO) mice confirmed that PDE10A operates downstream of nNOS to limit cGMP production and excitatory corticostriatal transmission. Thus, stimulation of PDE10A acts to attenuate corticostriatal transmission in a manner largely dependent on effects directed at the NO-sGC-cGMP signaling cascade.
Keywords: cGMP; medium-sized spiny neuron; nitric oxide; nitric oxide synthase; phosphodiesterase 10A; soluble guanylyl cyclase.
Copyright © 2015 the authors 0270-6474/15/355781-11$15.00/0.
Figures



Similar articles
-
Nitric oxide-soluble guanylyl cyclase signaling regulates corticostriatal transmission and short-term synaptic plasticity of striatal projection neurons recorded in vivo.Neuropharmacology. 2010 Mar;58(3):624-31. doi: 10.1016/j.neuropharm.2009.11.011. Epub 2009 Dec 5. Neuropharmacology. 2010. PMID: 19969007 Free PMC article.
-
Inhibition of Phosphodiesterase 10A Increases the Responsiveness of Striatal Projection Neurons to Cortical Stimulation.J Pharmacol Exp Ther. 2009 Mar;328(3):785-95. doi: 10.1124/jpet.108.146332. Epub 2008 Dec 4. J Pharmacol Exp Ther. 2009. PMID: 19056933 Free PMC article.
-
The nitric oxide-guanylyl cyclase signaling pathway modulates membrane activity States and electrophysiological properties of striatal medium spiny neurons recorded in vivo.J Neurosci. 2004 Feb 25;24(8):1924-35. doi: 10.1523/JNEUROSCI.4470-03.2004. J Neurosci. 2004. PMID: 14985433 Free PMC article.
-
Phosphodiesterase 10A inhibitors: a novel approach to the treatment of the symptoms of schizophrenia.Curr Opin Investig Drugs. 2007 Jan;8(1):54-9. Curr Opin Investig Drugs. 2007. PMID: 17263185 Review.
-
Regulation of Striatal Neuron Activity by Cyclic Nucleotide Signaling and Phosphodiesterase Inhibition: Implications for the Treatment of Parkinson's Disease.Adv Neurobiol. 2017;17:257-283. doi: 10.1007/978-3-319-58811-7_10. Adv Neurobiol. 2017. PMID: 28956336 Review.
Cited by
-
A Novel Role of Cyclic Nucleotide Phosphodiesterase 10A in Pathological Cardiac Remodeling and Dysfunction.Circulation. 2020 Jan 21;141(3):217-233. doi: 10.1161/CIRCULATIONAHA.119.042178. Epub 2019 Dec 5. Circulation. 2020. PMID: 31801360 Free PMC article.
-
Selective Effects of PDE10A Inhibitors on Striatopallidal Neurons Require Phosphatase Inhibition by DARPP-32.eNeuro. 2015 Aug 31;2(4):ENEURO.0060-15.2015. doi: 10.1523/ENEURO.0060-15.2015. eCollection 2015 Jul-Aug. eNeuro. 2015. PMID: 26465004 Free PMC article.
-
Targeting Striatal Glutamate and Phosphodiesterases to Control L-DOPA-Induced Dyskinesia.Cells. 2023 Nov 30;12(23):2754. doi: 10.3390/cells12232754. Cells. 2023. PMID: 38067182 Free PMC article. Review.
-
Structural insight into the lead identification of a dual inhibitor of PDE1B and PDE10A: Integrating pharmacophore-based virtual screening, molecular docking, and structure-activity-relationship approaches.Heliyon. 2024 Sep 23;10(19):e38305. doi: 10.1016/j.heliyon.2024.e38305. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39391487 Free PMC article.
-
Phosphodiesterase 9A Inhibition Facilitates Corticostriatal Transmission in Wild-Type and Transgenic Rats That Model Huntington's Disease.Front Neurosci. 2020 Jun 3;14:466. doi: 10.3389/fnins.2020.00466. eCollection 2020. Front Neurosci. 2020. PMID: 32581668 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
