Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117
- PMID: 25855300
- PMCID: PMC4890714
- DOI: 10.1038/nature14411
Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117
Erratum in
-
Corrigendum: Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117.Nature. 2016 Jul 28;535(7613):580. doi: 10.1038/nature17642. Epub 2016 Mar 23. Nature. 2016. PMID: 27007847 No abstract available.
Abstract
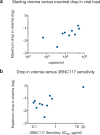
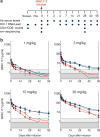
HIV-1 immunotherapy with a combination of first generation monoclonal antibodies was largely ineffective in pre-clinical and clinical settings and was therefore abandoned. However, recently developed single-cell-based antibody cloning methods have uncovered a new generation of far more potent broadly neutralizing antibodies to HIV-1 (refs 4, 5). These antibodies can prevent infection and suppress viraemia in humanized mice and nonhuman primates, but their potential for human HIV-1 immunotherapy has not been evaluated. Here we report the results of a first-in-man dose escalation phase 1 clinical trial of 3BNC117, a potent human CD4 binding site antibody, in uninfected and HIV-1-infected individuals. 3BNC117 infusion was well tolerated and demonstrated favourable pharmacokinetics. A single 30 mg kg(-1) infusion of 3BNC117 reduced the viral load in HIV-1-infected individuals by 0.8-2.5 log10 and viraemia remained significantly reduced for 28 days. Emergence of resistant viral strains was variable, with some individuals remaining sensitive to 3BNC117 for a period of 28 days. We conclude that, as a single agent, 3BNC117 is safe and effective in reducing HIV-1 viraemia, and that immunotherapy should be explored as a new modality for HIV-1 prevention, therapy and cure.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

