The effect of spinal cord injury on the neurochemical properties of vagal sensory neurons
- PMID: 25855310
- PMCID: PMC4469926
- DOI: 10.1152/ajpregu.00445.2014
The effect of spinal cord injury on the neurochemical properties of vagal sensory neurons
Abstract
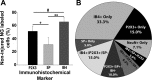
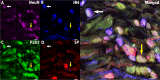
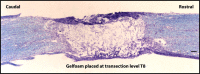
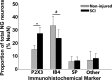
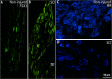
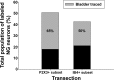
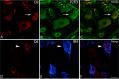
The vagus nerve is composed primarily of nonmyelinated sensory neurons whose cell bodies are located in the nodose ganglion (NG). The vagus has widespread projections that supply most visceral organs, including the bladder. Because of its nonspinal route, the vagus nerve itself is not directly damaged from spinal cord injury (SCI). Because most viscera, including bladder, are dually innervated by spinal and vagal sensory neurons, an impact of SCI on the sensory component of vagal circuitry may contribute to post-SCI visceral pathologies. To determine whether SCI, in male Wistar rats, might impact neurochemical characteristics of NG neurons, immunohistochemical assessments were performed for P2X3 receptor expression, isolectin B4 (IB4) binding, and substance P expression, three known injury-responsive markers in sensory neuronal subpopulations. In addition to examining the overall population of NG neurons, those innervating the urinary bladder also were assessed separately. All three of the molecular markers were represented in the NG from noninjured animals, with the majority of the neurons binding IB4. In the chronically injured rats, there was a significant increase in the number of NG neurons expressing P2X3 and a significant decrease in the number binding IB4 compared with noninjured animals, a finding that held true also for the bladder-innervating population. Overall, these results indicate that vagal afferents, including those innervating the bladder, display neurochemical plasticity post-SCI that may have implications for visceral homeostatic mechanisms and nociceptive signaling.
Keywords: bladder; immunohistochemical phenotype; nodose ganglion; spinal cord injury; vagus nerve.
Copyright © 2015 the American Physiological Society.
Figures

References
-
- Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989. - PubMed
-
- Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104: 502–509, 1993. - PubMed
-
- Amir R, Devor M. Functional cross-excitation between afferent A- and C-neurons in dorsal root ganglia. Neuroscience 95: 189–195, 2000. - PubMed
-
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21: 1371–1383, 2004. - PubMed
-
- Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol 284: F966–F976, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

