Cadherin flexibility provides a key difference between desmosomes and adherens junctions
- PMID: 25855637
- PMCID: PMC4418904
- DOI: 10.1073/pnas.1420508112
Cadherin flexibility provides a key difference between desmosomes and adherens junctions
Abstract
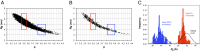
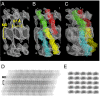
Desmosomes and adherens junctions are intercellular adhesive structures essential for the development and integrity of vertebrate tissue, including the epidermis and heart. Their cell adhesion molecules are cadherins: type 1 cadherins in adherens junctions and desmosomal cadherins in desmosomes. A fundamental difference is that desmosomes have a highly ordered structure in their extracellular region and exhibit calcium-independent hyperadhesion, whereas adherens junctions appear to lack such ordered arrays, and their adhesion is always calcium-dependent. We present here the structure of the entire ectodomain of desmosomal cadherin desmoglein 2 (Dsg2), using a combination of small-angle X-ray scattering, electron microscopy, and solution-based biophysical techniques. This structure reveals that the ectodomain of Dsg2 is flexible even in the calcium-bound state and, on average, is shorter than the type 1 cadherin crystal structures. The Dsg2 structure has an excellent fit with the electron tomography reconstructions of human desmosomes. This fit suggests an arrangement in which desmosomal cadherins form trans interactions but are too far apart to interact in cis, in agreement with previously reported observations. Cadherin flexibility may be key to explaining the plasticity of desmosomes that maintain tissue integrity in their hyperadhesive form, but can adopt a weaker, calcium-dependent adhesion during wound healing and early development.
Keywords: adhesion; cadherins; desmosomes; small-angle X-ray scattering; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ivanov AI, Naydenov NG. Dynamics and regulation of epithelial adherens junctions: Recent discoveries and controversies. Int Rev Cell Mol Biol. 2013;303:27–99. - PubMed
-
- Boggon TJ, et al. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296(5571):1308–1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

