The expanding genetic toolbox of the wasp Nasonia vitripennis and its relatives
- PMID: 25855650
- PMCID: PMC4391559
- DOI: 10.1534/genetics.112.147512
The expanding genetic toolbox of the wasp Nasonia vitripennis and its relatives
Abstract
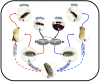
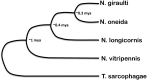
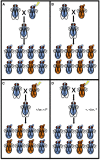
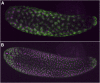
The parasitoid wasp Nasonia represents a genus of four species that is emerging as a powerful genetic model system that has made and will continue to make important contributions to our understanding of evolutionary biology, development, ecology, and behavior. Particularly powerful are the haplodiploid genetics of the system, which allow some of the advantages of microbial genetics to be applied to a complex multicellular eukaryote. In addition, fertile, viable hybrids can be made among the four species in the genus. This makes Nasonia exceptionally well suited for evolutionary genetics approaches, especially when combined with its haploid genetics and tractability in the laboratory. These features are complemented by an expanding array of genomic, transcriptomic, and functional resources, the application of which has already made Nasonia an important model system in such emerging fields as evolutionary developmental biology and microbiomics. This article describes the genetic and genomic advantages of Nasonia wasps and the resources available for their genetic analysis.
Keywords: Nasonia; genomics; haplodiploid; hybrid genetics; model organisms.
Copyright © 2015 by the Genetics Society of America.
Figures
Similar articles
-
The parasitoid wasp Nasonia: an emerging model system with haploid male genetics.Cold Spring Harb Protoc. 2009 Oct;2009(10):pdb.emo134. doi: 10.1101/pdb.emo134. Cold Spring Harb Protoc. 2009. PMID: 20147035 Free PMC article.
-
Fine-scale mapping of the Nasonia genome to chromosomes using a high-density genotyping microarray.G3 (Bethesda). 2013 Feb;3(2):205-15. doi: 10.1534/g3.112.004739. Epub 2013 Feb 1. G3 (Bethesda). 2013. PMID: 23390597 Free PMC article.
-
Dissection of the complex genetic basis of craniofacial anomalies using haploid genetics and interspecies hybrids in Nasonia wasps.Dev Biol. 2016 Jul 15;415(2):391-405. doi: 10.1016/j.ydbio.2015.12.022. Epub 2015 Dec 23. Dev Biol. 2016. PMID: 26721604 Free PMC article.
-
The jewel wasp Nasonia: querying the genome with haplo-diploid genetics.Genesis. 2003 Mar;35(3):185-91. doi: 10.1002/gene.10189. Genesis. 2003. PMID: 12640624 Review.
-
Nasonia-microbiome associations: a model for evolutionary hologenomics research.Trends Parasitol. 2023 Feb;39(2):101-112. doi: 10.1016/j.pt.2022.11.005. Epub 2022 Dec 7. Trends Parasitol. 2023. PMID: 36496327 Review.
Cited by
-
WaspAtlas: a Nasonia vitripennis gene database and analysis platform.Database (Oxford). 2015 Oct 9;2015:bav103. doi: 10.1093/database/bav103. Print 2015. Database (Oxford). 2015. PMID: 26452372 Free PMC article.
-
Disentangling a Holobiont - Recent Advances and Perspectives in Nasonia Wasps.Front Microbiol. 2016 Sep 23;7:1478. doi: 10.3389/fmicb.2016.01478. eCollection 2016. Front Microbiol. 2016. PMID: 27721807 Free PMC article. Review.
-
Genome Report: Whole Genome Sequence and Annotation of the Parasitoid Jewel Wasp Nasonia giraulti Laboratory Strain RV2X[u].G3 (Bethesda). 2020 Aug 5;10(8):2565-2572. doi: 10.1534/g3.120.401200. G3 (Bethesda). 2020. PMID: 32571804 Free PMC article.
-
Genome and Ontogenetic-Based Transcriptomic Analyses of the Flesh Fly, Sarcophaga bullata.G3 (Bethesda). 2019 May 7;9(5):1313-1320. doi: 10.1534/g3.119.400148. G3 (Bethesda). 2019. PMID: 30926723 Free PMC article.
-
Expression and Function of Toll Pathway Components in the Early Development of the Wasp Nasonia vitripennis.J Dev Biol. 2022 Jan 26;10(1):7. doi: 10.3390/jdb10010007. J Dev Biol. 2022. PMID: 35225961 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

