P2Y12 Receptor Localizes in the Renal Collecting Duct and Its Blockade Augments Arginine Vasopressin Action and Alleviates Nephrogenic Diabetes Insipidus
- PMID: 25855780
- PMCID: PMC4657822
- DOI: 10.1681/ASN.2014010118
P2Y12 Receptor Localizes in the Renal Collecting Duct and Its Blockade Augments Arginine Vasopressin Action and Alleviates Nephrogenic Diabetes Insipidus
Abstract
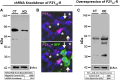
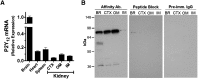
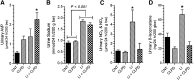
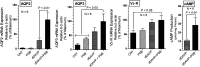
P2Y12 receptor (P2Y12-R) signaling is mediated through Gi, ultimately reducing cellular cAMP levels. Because cAMP is a central modulator of arginine vasopressin (AVP)-induced water transport in the renal collecting duct (CD), we hypothesized that if expressed in the CD, P2Y12-R may play a role in renal handling of water in health and in nephrogenic diabetes insipidus. We found P2Y12-R mRNA expression in rat kidney, and immunolocalized its protein and aquaporin-2 (AQP2) in CD principal cells. Administration of clopidogrel bisulfate, an irreversible inhibitor of P2Y12-R, significantly increased urine concentration and AQP2 protein in the kidneys of Sprague-Dawley rats. Notably, clopidogrel did not alter urine concentration in Brattleboro rats that lack AVP. Clopidogrel administration also significantly ameliorated lithium-induced polyuria, improved urine concentrating ability and AQP2 protein abundance, and reversed the lithium-induced increase in free-water excretion, without decreasing blood or kidney tissue lithium levels. Clopidogrel administration also augmented the lithium-induced increase in urinary AVP excretion and suppressed the lithium-induced increase in urinary nitrates/nitrites (nitric oxide production) and 8-isoprostane (oxidative stress). Furthermore, selective blockade of P2Y12-R by the reversible antagonist PSB-0739 in primary cultures of rat inner medullary CD cells potentiated the expression of AQP2 and AQP3 mRNA, and cAMP production induced by dDAVP (desmopressin). In conclusion, pharmacologic blockade of renal P2Y12-R increases urinary concentrating ability by augmenting the effect of AVP on the kidney and ameliorates lithium-induced NDI by potentiating the action of AVP on the CD. This strategy may offer a novel and effective therapy for lithium-induced NDI.
Keywords: collecting ducts; cyclic AMP; diabetes insipidus; extracellular; hypothalamus; nucleotides; vasopressin.
Copyright © 2015 by the American Society of Nephrology.
Figures




References
-
- Olesen ET, Fenton RA: Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol 24: 169–178, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

