Hyaluronidase Hyal1 Increases Tumor Cell Proliferation and Motility through Accelerated Vesicle Trafficking
- PMID: 25855794
- PMCID: PMC4505569
- DOI: 10.1074/jbc.M115.647446
Hyaluronidase Hyal1 Increases Tumor Cell Proliferation and Motility through Accelerated Vesicle Trafficking
Abstract
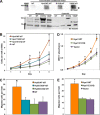
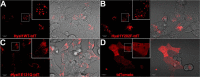
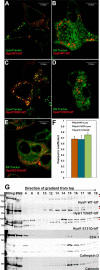
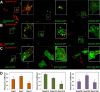
Hyaluronan (HA) turnover accelerates metastatic progression of prostate cancer in part by increasing rates of tumor cell proliferation and motility. To determine the mechanism, we overexpressed hyaluronidase 1 (Hyal1) as a fluorescent fusion protein and examined its impact on endocytosis and vesicular trafficking. Overexpression of Hyal1 led to increased rates of internalization of HA and the endocytic recycling marker transferrin. Live imaging of Hyal1, sucrose gradient centrifugation, and specific colocalization of Rab GTPases defined the subcellular distribution of Hyal1 as early and late endosomes, lysosomes, and recycling vesicles. Manipulation of vesicular trafficking by chemical inhibitors or with constitutively active and dominant negative Rab expression constructs caused atypical localization of Hyal1. Using the catalytically inactive point mutant Hyal1-E131Q, we found that enzymatic activity of Hyal1 was necessary for normal localization within the cell as Hyal1-E131Q was mainly detected within the endoplasmic reticulum. Expression of a HA-binding point mutant, Hyal1-Y202F, revealed that secretion of Hyal1 and concurrent reuptake from the extracellular space are critical for rapid HA internalization and cell proliferation. Overall, excess Hyal1 secretion accelerates endocytic vesicle trafficking in a substrate-dependent manner, promoting aggressive tumor cell behavior.
Keywords: cell motility; endocytosis; hyaluronan; hyaluronidase; lysosome; prostate cancer.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Siegel R., Ma J., Zou Z., Jemal A. (2014) Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 - PubMed
-
- Aaltomaa S., Lipponen P., Tammi R., Tammi M., Viitanen J., Kankkunen J. P., Kosma V. M. (2002) Strong stromal hyaluronan expression is associated with PSA recurrence in local prostate cancer. Urol. Int. 69, 266–272 - PubMed
-
- Anttila M. A., Tammi R. H., Tammi M. I., Syrjänen K. J., Saarikoski S. V., Kosma V. M. (2000) High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 60, 150–155 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

