Vitreal delivery of AAV vectored Cnga3 restores cone function in CNGA3-/-/Nrl-/- mice, an all-cone model of CNGA3 achromatopsia
- PMID: 25855802
- PMCID: PMC4459390
- DOI: 10.1093/hmg/ddv114
Vitreal delivery of AAV vectored Cnga3 restores cone function in CNGA3-/-/Nrl-/- mice, an all-cone model of CNGA3 achromatopsia
Abstract
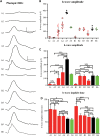
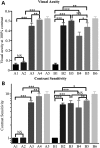
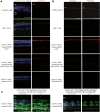
The CNGA3(-/-)/Nrl(-/-) mouse is a cone-dominant model with Cnga3 channel deficiency, which partially mimics the all cone foveal structure of human achromatopsia 2 with CNGA3 mutations. Although subretinal (SR) AAV vector administration can transfect retinal cells efficiently, the injection-induced retinal detachment can cause retinal damage, particularly when SR vector bleb includes the fovea. We therefore explored whether cone function-structure could be rescued in CNGA3(-/-)/Nrl(-/-) mice by intravitreal (IVit) delivery of tyrosine to phenylalanine (Y-F) capsid mutant AAV8. We find that AAV-mediated CNGA3 expression can restore cone function and rescue structure following IVit delivery of AAV8 (Y447, 733F) vector. Rescue was assessed by restoration of the cone-mediated electroretinogram (ERG), optomotor responses, and cone opsin immunohistochemistry. Demonstration of gene therapy in a cone-dominant mouse model by IVit delivery provides a potential alternative vector delivery mode for safely transducing foveal cones in achromatopsia patients and in other human retinal diseases affecting foveal function.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Long-term retinal cone rescue using a capsid mutant AAV8 vector in a mouse model of CNGA3-achromatopsia.PLoS One. 2017 Nov 13;12(11):e0188032. doi: 10.1371/journal.pone.0188032. eCollection 2017. PLoS One. 2017. PMID: 29131863 Free PMC article.
-
Gene Augmentation Therapy Restores Retinal Function and Visual Behavior in a Sheep Model of CNGA3 Achromatopsia.Mol Ther. 2015 Sep;23(9):1423-33. doi: 10.1038/mt.2015.114. Epub 2015 Jun 19. Mol Ther. 2015. PMID: 26087757 Free PMC article.
-
Safety and Efficacy Evaluation of rAAV2tYF-PR1.7-hCNGA3 Vector Delivered by Subretinal Injection in CNGA3 Mutant Achromatopsia Sheep.Hum Gene Ther Clin Dev. 2017 Jun;28(2):96-107. doi: 10.1089/humc.2017.028. Epub 2017 May 5. Hum Gene Ther Clin Dev. 2017. PMID: 28478700
-
Gene Therapy for Color Blindness.Yale J Biol Med. 2017 Dec 19;90(4):543-551. eCollection 2017 Dec. Yale J Biol Med. 2017. PMID: 29259520 Free PMC article. Review.
-
[Gene replacement therapy in achromatopsia type 2].Klin Monbl Augenheilkd. 2014 Mar;231(3):232-40. doi: 10.1055/s-0034-1368180. Epub 2014 Mar 21. Klin Monbl Augenheilkd. 2014. PMID: 24658860 Review. German.
Cited by
-
Retinal Degeneration Caused by Ago2 Disruption.Invest Ophthalmol Vis Sci. 2021 Sep 2;62(12):14. doi: 10.1167/iovs.62.12.14. Invest Ophthalmol Vis Sci. 2021. PMID: 34529004 Free PMC article.
-
The Degeneration and Apoptosis Patterns of Cone Photoreceptors in rd11 Mice.J Ophthalmol. 2017;2017:9721362. doi: 10.1155/2017/9721362. Epub 2017 Jan 12. J Ophthalmol. 2017. PMID: 28168050 Free PMC article.
-
Residual Foveal Cone Structure in CNGB3-Associated Achromatopsia.Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):3984-95. doi: 10.1167/iovs.16-19313. Invest Ophthalmol Vis Sci. 2016. PMID: 27479814 Free PMC article.
-
Animal modelling for inherited central vision loss.J Pathol. 2016 Jan;238(2):300-10. doi: 10.1002/path.4641. Epub 2015 Nov 13. J Pathol. 2016. PMID: 26387748 Free PMC article. Review.
-
Retinal Cyclic Nucleotide-Gated Channels: From Pathophysiology to Therapy.Int J Mol Sci. 2018 Mar 7;19(3):749. doi: 10.3390/ijms19030749. Int J Mol Sci. 2018. PMID: 29518895 Free PMC article. Review.
References
-
- Kohl S., Varsanyi B., Antunes G.A., Baumann B., Hoyng C.B., Jagle H., Rosenberg T., Kellner U., Lorenz B., Salati R., et al. (2005) CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur. J. Hum. Genet., 13, 302–308. - PubMed
-
- Kaupp U.B., Seifert R. (2002) Cyclic nucleotide-gated ion channels. Physiol. Rev., 82, 769–824. - PubMed
-
- Zelinger L., Greenberg A., Kohl S., Banin E., Sharon D. (2010) An ancient autosomal haplotype bearing a rare achromatopsia-causing founder mutation is shared among Arab Muslims and Oriental Jews. Hum. Genet., 128, 261–267. - PubMed
-
- Dai X.F., Pang J.J. (2012) [Progress on study of achromatopsia and targeted gene therapy]. Zhonghua Yan Ke Za Zhi, 48, 755–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

