2'-Fluoro-modified phosphorothioate oligonucleotide can cause rapid degradation of P54nrb and PSF
- PMID: 25855809
- PMCID: PMC4482069
- DOI: 10.1093/nar/gkv298
2'-Fluoro-modified phosphorothioate oligonucleotide can cause rapid degradation of P54nrb and PSF
Abstract
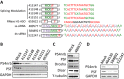
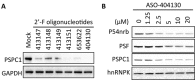
Synthetic oligonucleotides are used to regulate gene expression through different mechanisms. Chemical modifications of the backbone of the nucleic acid and/or of the 2' moiety of the ribose can increase nuclease stability and/or binding affinity of oligonucleotides to target molecules. Here we report that transfection of 2'-F-modified phosphorothioate oligonucleotides into cells can reduce the levels of P54nrb and PSF proteins through proteasome-mediated degradation. Such deleterious effects of 2'-F-modified oligonucleotides were observed in different cell types from different species, and were independent of oligonucleotide sequence, positions of the 2'-F-modified nucleotides in the oligonucleotides, method of delivery or mechanism of action of the oligonucleotides. Four 2'-F-modified nucleotides were sufficient to cause the protein reduction. P54nrb and PSF belong to Drosophila behavior/human splicing (DBHS) family. The third member of the family, PSPC1, was also reduced by the 2'-F-modified oligonucleotides. Preferential association of 2'-F-modified oligonucleotides with P54nrb was observed, which is partially responsible for the protein reduction. Consistent with the role of DBHS proteins in double-strand DNA break (DSB) repair, elevated DSBs were observed in cells treated with 2'-F-modified oligonucleotides, which contributed to severe impairment in cell proliferation. These results suggest that oligonucleotides with 2'-F modifications can cause non-specific loss of cellular protein(s).
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Crooke S.T., Vickers T., Lima W., Wu H.-J. Mechanisms of antisense drug action, an introduction. In: Crooke ST, editor. Antisense Drug Technology—Principles, Strategies, and Application. Boca Raton, FL: CRC Press; 2006. pp. 2273–2304.
-
- Bennett C.F., Swayze E.E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

