Peroxisome proliferator-activated receptorβ/δ activation is essential for modulating p-Foxo1/Foxo1 status in functional insulin-positive cell differentiation
- PMID: 25855963
- PMCID: PMC4650555
- DOI: 10.1038/cddis.2015.88
Peroxisome proliferator-activated receptorβ/δ activation is essential for modulating p-Foxo1/Foxo1 status in functional insulin-positive cell differentiation
Abstract
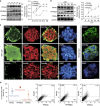
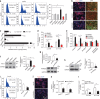
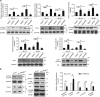
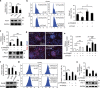
Peroxisome proliferator-activated receptors (PPARs) participate in energy homeostasis and play essential roles in diabetes therapy through their effects on non-pancreas tissues. Pathological microenvironment may influence the metabolic requirements for the maintenance of stem cell differentiation. Accordingly, understanding the mechanisms of PPARs on pancreatic β-cell differentiation may be helpful to find the underlying targets of disrupted energy homeostasis under the pancreatic disease condition. PPARs are involved in stem cell differentiation via mitochondrial oxidative phosphorylation, but the subtype member activation and the downstream regulation in functional insulin-positive (INS+) cell differentiation remain unclear. Here, we show a novel role of PPARβ/δ activation in determining INS+ cell differentiation and functional maturation. We found PPARβ/δ expression selectively upregulated in mouse embryonic pancreases or stem cells-derived INS+ cells at the pancreatic mature stage in vivo and in vitro. Strikingly, given the inefficiency of generating INS+ cells in vitro, PPARβ/δ activation displayed increasing mouse and human ES cell-derived INS+ cell numbers and insulin secretion. This phenomenon was closely associated with the forkhead box protein O1 (Foxo1) nuclear shuttling, which was dependent on PPARβ/δ downstream PI3K/Akt signaling transduction. The present study reveals the essential role of PPARβ/δ activation on p-Foxo1/Foxo1 status, and in turn, determining INS+ cell generation and insulin secretion via affecting pancreatic and duodenal homeobox-1 expression. The results demonstrate the underlying mechanism by which PPARβ/δ activation promotes functional INS+ cell differentiation. It also provides potential targets for anti-diabetes drug discovery and hopeful clinical applications in human cell therapy.
Figures

Similar articles
-
Activation of PPARβ/δ protects pancreatic β cells from palmitate-induced apoptosis by upregulating the expression of GLP-1 receptor.Cell Signal. 2014 Feb;26(2):268-78. doi: 10.1016/j.cellsig.2013.11.019. Epub 2013 Nov 21. Cell Signal. 2014. PMID: 24269940
-
FoxO1 inhibition promotes differentiation of human embryonic stem cells into insulin producing cells.Exp Cell Res. 2018 Jan 1;362(1):227-234. doi: 10.1016/j.yexcr.2017.11.022. Epub 2017 Nov 20. Exp Cell Res. 2018. PMID: 29157981
-
Forkhead box O1/pancreatic and duodenal homeobox 1 intracellular translocation is regulated by c-Jun N-terminal kinase and involved in prostaglandin E2-induced pancreatic beta-cell dysfunction.Endocrinology. 2009 Dec;150(12):5284-93. doi: 10.1210/en.2009-0671. Epub 2009 Oct 16. Endocrinology. 2009. PMID: 19837872
-
Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) acts as regulator of metabolism linked to multiple cellular functions.Pharmacol Ther. 2010 Mar;125(3):423-35. doi: 10.1016/j.pharmthera.2009.12.001. Epub 2009 Dec 22. Pharmacol Ther. 2010. PMID: 20026355 Review.
-
Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation.Pharmacol Ther. 2009 Nov;124(2):141-50. doi: 10.1016/j.pharmthera.2009.06.011. Epub 2009 Jul 15. Pharmacol Ther. 2009. PMID: 19615407 Review.
Cited by
-
FGF10 Enhances Peripheral Nerve Regeneration via the Preactivation of the PI3K/Akt Signaling-Mediated Antioxidant Response.Front Pharmacol. 2019 Oct 16;10:1224. doi: 10.3389/fphar.2019.01224. eCollection 2019. Front Pharmacol. 2019. PMID: 31680984 Free PMC article.
-
PPARs and the Development of Type 1 Diabetes.PPAR Res. 2020 Jan 9;2020:6198628. doi: 10.1155/2020/6198628. eCollection 2020. PPAR Res. 2020. PMID: 32395123 Free PMC article. Review.
-
Endothelial Foxo1 Phosphorylation Inhibition via Aptamer-Liposome Alleviates OPN-Induced Pathological Vascular Remodeling Following Spinal Cord Injury.Adv Sci (Weinh). 2024 Nov;11(43):e2406398. doi: 10.1002/advs.202406398. Epub 2024 Sep 28. Adv Sci (Weinh). 2024. PMID: 39340832 Free PMC article.
-
Chiglitazar ameliorates dehydroepiandrosterone-induced polycystic ovary syndrome in rats.J Ovarian Res. 2024 Nov 19;17(1):229. doi: 10.1186/s13048-024-01554-6. J Ovarian Res. 2024. PMID: 39563391 Free PMC article.
-
Acute binge alcohol alters whole body metabolism and the time-dependent expression of skeletal muscle-specific metabolic markers for multiple days in mice.Am J Physiol Endocrinol Metab. 2022 Sep 1;323(3):E215-E230. doi: 10.1152/ajpendo.00026.2022. Epub 2022 Jul 6. Am J Physiol Endocrinol Metab. 2022. PMID: 35793479 Free PMC article.
References
-
- 2Docherty K, Bernardo AS, Vallier L. Embryonic stem cell therapy for diabetes mellitus. Semin Cell Dev Biol 2007; 18: 827–838. - PubMed
-
- 3Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O'Neil JJ et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 2013; 31: 2432–2442. - PubMed
-
- 4Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001; 292: 1389–1394. - PubMed
-
- 5D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006; 24: 1392–1401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

