Perilipin1 deficiency in whole body or bone marrow-derived cells attenuates lesions in atherosclerosis-prone mice
- PMID: 25855981
- PMCID: PMC4391836
- DOI: 10.1371/journal.pone.0123738
Perilipin1 deficiency in whole body or bone marrow-derived cells attenuates lesions in atherosclerosis-prone mice
Abstract
Aims: The objective of this study is to determine the role of perilipin 1 (Plin1) in whole body or bone marrow-derived cells on atherogenesis.
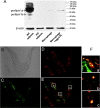
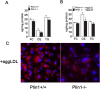
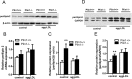
Methods and results: Accumulated evidence have indicated the role of Plin1 in atherosclerosis, however, these findings are controversial. In this study, we showed that Plin1 was assembled and colocalized with CD68 in macrophages in atherosclerotic plaques of ApoE-/- mice. We further found 39% reduction of plaque size in the aortic roots of Plin1 and ApoE double knockout (Plin1-/-ApoE-/-) females compared with ApoE-/- female littermates. In order to verify whether this reduction was macrophage-specific, the bone marrow cells from wild-type or Plin1 deficient mice (Plin1-/-) were transplanted into LDL receptor deficient mice (LDLR-/-). Mice receiving Plin1-/- bone marrow cells showed also 49% reduction in aortic atherosclerotic lesions compared with LDLR-/- mice received wild-type bone marrow cells. In vitro experiments showed that Plin1-/- macrophages had decreased protein expression of CD36 translocase and an enhanced cholesterol ester hydrolysis upon aggregated-LDL loading, with unaltered expression of many other regulators of cholesterol metabolism, such as cellular lipases, and Plin2 and 3. Given the fundamental role of Plin1 in protecting LD lipids from lipase hydrolysis, it is reasonably speculated that the assembly of Plin1 in microphages might function to reduce lipolysis and hence increase lipid retention in ApoE-/- plaques, but this pro-atherosclerotic property would be abrogated on inactivation of Plin1.
Conclusion: Plin1 deficiency in bone marrow-derived cells may be responsible for reduced atherosclerotic lesions in the mice.
Conflict of interest statement
Figures



Similar articles
-
Increased atherosclerosis in mice deficient in perilipin1.Lipids Health Dis. 2011 Sep 24;10:169. doi: 10.1186/1476-511X-10-169. Lipids Health Dis. 2011. PMID: 21943217 Free PMC article.
-
Orp8 deficiency in bone marrow-derived cells reduces atherosclerotic lesion progression in LDL receptor knockout mice.PLoS One. 2014 Oct 27;9(10):e109024. doi: 10.1371/journal.pone.0109024. eCollection 2014. PLoS One. 2014. PMID: 25347070 Free PMC article.
-
Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency.Arterioscler Thromb Vasc Biol. 2014 May;34(5):976-84. doi: 10.1161/ATVBAHA.113.303097. Epub 2014 Mar 20. Arterioscler Thromb Vasc Biol. 2014. PMID: 24651678 Free PMC article.
-
Perilipin 1: a systematic review on its functions on lipid metabolism and atherosclerosis in mice and humans.Cardiovasc Res. 2024 Mar 14;120(3):237-248. doi: 10.1093/cvr/cvae005. Cardiovasc Res. 2024. PMID: 38214891
-
Lipid droplet associated proteins: an emerging role in atherogenesis.Histol Histopathol. 2011 May;26(5):631-42. doi: 10.14670/HH-26.631. Histol Histopathol. 2011. PMID: 21432779 Review.
Cited by
-
Lipid droplet-associated proteins in atherosclerosis (Review).Mol Med Rep. 2016 Jun;13(6):4527-34. doi: 10.3892/mmr.2016.5099. Epub 2016 Apr 11. Mol Med Rep. 2016. PMID: 27082419 Free PMC article. Review.
-
Rare coding variants in 35 genes associate with circulating lipid levels-A multi-ancestry analysis of 170,000 exomes.Am J Hum Genet. 2022 Jan 6;109(1):81-96. doi: 10.1016/j.ajhg.2021.11.021. Epub 2021 Dec 20. Am J Hum Genet. 2022. PMID: 34932938 Free PMC article.
-
Cardiotonic Pills Plus Recombinant Human Prourokinase Ameliorates Atherosclerotic Lesions in LDLR-/- Mice.Front Physiol. 2019 Sep 10;10:1128. doi: 10.3389/fphys.2019.01128. eCollection 2019. Front Physiol. 2019. PMID: 31551808 Free PMC article.
-
Hydrogen sulfide lowers hyperhomocysteinemia dependent on cystathionine γ lyase S-sulfhydration in ApoE-knockout atherosclerotic mice.Br J Pharmacol. 2019 Sep;176(17):3180-3192. doi: 10.1111/bph.14719. Epub 2019 Jul 14. Br J Pharmacol. 2019. PMID: 31140595 Free PMC article.
-
Differences between the Molecular Mechanisms Underlying Ruptured and Non-Ruptured Carotid Plaques, and the Significance of ABCA1.J Stroke. 2018 Jan;20(1):80-91. doi: 10.5853/jos.2017.02390. Epub 2018 Jan 31. J Stroke. 2018. PMID: 29402067 Free PMC article.
References
-
- Zweytick D, Athenstaedt K, Daum G. Intracellular lipid particles of eukaryotic cells. Biochim Biophys Acta. 2000; 1469: 101–120. - PubMed
-
- Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006; 7: 373–378. - PubMed
-
- Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995; 57: 791–804. - PubMed
-
- Larigauderie G, Bouhlel MA, Furman C, Jaye M, Fruchart JC, Rouis M. Perilipin, a potential substitute for adipophilin in triglyceride storage in human macrophages. Atherosclerosis. 2006; 189: 142–148. - PubMed
-
- Buechler C, Ritter M, Duong CQ, Orso E, Kapinsky M, Schmitz G. Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim Biophys Acta. 2001; 1532: 97–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

