MiRNA-615-5p functions as a tumor suppressor in pancreatic ductal adenocarcinoma by targeting AKT2
- PMID: 25856297
- PMCID: PMC4391776
- DOI: 10.1371/journal.pone.0119783
MiRNA-615-5p functions as a tumor suppressor in pancreatic ductal adenocarcinoma by targeting AKT2
Erratum in
-
Correction: MiRNA-615-5p Functions as a Tumor Suppressor in Pancreatic Ductal Adenocarcinoma by Targeting AKT2.PLoS One. 2015 May 8;10(5):e0128257. doi: 10.1371/journal.pone.0128257. eCollection 2015. PLoS One. 2015. PMID: 25955002 Free PMC article. No abstract available.
Abstract
Background: Aberrant microRNA (miRNA) expression is associated with tumor development. This study aimed to elucidate the role of miR-615-5p in the development of pancreatic ductal adenocarcinoma (PDAC).
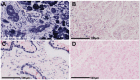
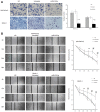
Methods: Locked nucleic acid in situ hybridization (LNA-ISH) was performed to compare miR-615-5p expression in patients between PDAC and matched adjacent normal tissues. Effects of miR-615-5p overexpression on cell proliferation, apoptosis, colony formation, migration, and invasion were determined in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Effects of miR-615-5p on AKT2 were examined by dual-luciferase reporter assay. Lentivirus expressing miR-615 was used to create stable overexpression cell lines, which were subsequently used in mouse xenograft and metastasis models to assess tumor growth, apoptosis and metastasis.
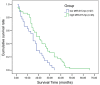
Results: miR-615-5p expression was significantly lower in PDAC than in adjacent normal tissues. Low levels of miR-615-5p were independently associated with poor prognosis (HR: 2.243, 95% CI: 1.190-4.227, P=0.013). AKT2 protein expression was inversely correlated with miR-615-5p expression (r=-0.3, P=0.003). miR-615-5p directly targeted the 3'-untranslated region of AKT2 mRNA and repressed its expression. miR-615-5p overexpression inhibited pancreatic cancer cell proliferation, migration, and invasion in vitro, and tumor growth and metastasis in vivo. Furthermore, miR-615-5p overexpression also induced pancreatic cancer cell apoptosis both in vitro and in vivo.
Conclusions: These results show that miR-615-5p inhibits pancreatic cancer cell proliferation, migration, and invasion by targeting AKT2. The data implicate miR-615-5p in the prognosis and treatment of PDAC.
Conflict of interest statement
Figures




References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma, v.1. Available: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 2015 Feb 3.
-
- Fuchs CS, Colditz GA, Stampfer MJ, Giovannucci EL, Hunter DJ, Rimm EB, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156: 2255–2260. - PubMed
-
- Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L and Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283: 2552–2558. - PubMed
-
- Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ and Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286: 921–929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

