In vitro microtumors provide a physiologically predictive tool for breast cancer therapeutic screening
- PMID: 25856378
- PMCID: PMC4391795
- DOI: 10.1371/journal.pone.0123312
In vitro microtumors provide a physiologically predictive tool for breast cancer therapeutic screening
Abstract
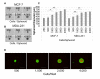
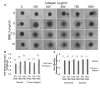
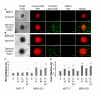
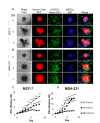
Many anti-cancer drugs fail in human trials despite showing efficacy in preclinical models. It is clear that the in vitro assays involving 2D monoculture do not reflect the complex extracellular matrix, chemical, and cellular microenvironment of the tumor tissue, and this may explain the failure of 2D models to predict clinical efficacy. We first optimized an in vitro microtumor model using a tumor-aligned ECM, a tumor-aligned medium, MCF-7 and MDA-MB-231 breast cancer spheroids, human umbilical vein endothelial cells, and human stromal cells to recapitulate the tissue architecture, chemical environment, and cellular organization of a growing and invading tumor. We assayed the microtumor for cell proliferation and invasion in a tumor-aligned extracellular matrix, exhibiting collagen deposition, acidity, glucose deprivation, and hypoxia. We found maximal proliferation and invasion when the multicellular spheroids were cultured in a tumor-aligned medium, having low pH and low glucose, with 10% fetal bovine serum under hypoxic conditions. In a 7-day assay, varying doses of fluorouracil or paclitaxel had differential effects on proliferation for MCF-7 and MDA-MB-231 tumor spheroids in microtumor compared to 2D and 3D monoculture. The microtumors exhibited a tumor morphology and drug response similar to published xenograft data, thus demonstrating a more physiologically predictive in vitro model.
Conflict of interest statement
Figures

References
-
- Herper M. The Cost Of Creating A New Drug Now $5 Billion, Pushing Big Pharma To Change. Forbes. [Magazine]. 2013 8/11/2013(http://www.forbes.com/sites/matthewherper/2013/08/11/how-the-staggering-...).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

