Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration
- PMID: 25856413
- PMCID: PMC4534325
- DOI: 10.1002/glia.22835
Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration
Abstract
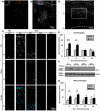
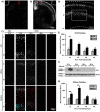
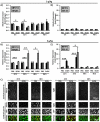
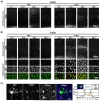
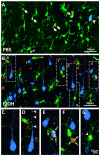
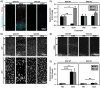
Fetal alcohol exposure is the most common known cause of preventable mental retardation, yet we know little about how microglia respond to, or are affected by, alcohol in the developing brain in vivo. Using an acute (single day) model of moderate (3 g/kg) to severe (5 g/kg) alcohol exposure in postnatal day (P) 7 or P8 mice, we found that alcohol-induced neuroapoptosis in the neocortex is closely correlated in space and time with the appearance of activated microglia near dead cells. The timing and molecular pattern of microglial activation varied with the level of cell death. Although microglia rapidly mobilized to contact and engulf late-stage apoptotic neurons, apoptotic bodies temporarily accumulated in neocortex, suggesting that in severe cases of alcohol toxicity the neurodegeneration rate exceeds the clearance capacity of endogenous microglia. Nevertheless, most dead cells were cleared and microglia began to deactivate within 1-2 days of the initial insult. Coincident with microglial activation and deactivation, there was a transient increase in expression of pro-inflammatory factors, TNFα and IL-1β, after severe (5 g/kg) but not moderate (3 g/kg) EtOH levels. Alcohol-induced microglial activation and pro-inflammatory factor expression were largely abolished in BAX null mice lacking neuroapoptosis, indicating that microglial activation is primarily triggered by apoptosis rather than the alcohol. Therefore, acute alcohol exposure in the developing neocortex causes transient microglial activation and mobilization, promoting clearance of dead cells and tissue recovery. Moreover, cortical microglia show a remarkable capacity to rapidly deactivate following even severe neurodegenerative insults in the developing brain.
Keywords: apoptosis; development; fetal alcohol spectrum disorders; phagocytosis.
© 2015 Wiley Periodicals, Inc.
Figures


References
-
- Adayev T, Estephan R, Meserole S, Mazza B, Yurkow EJ, Banerjee P. Externalization of phosphatidylserine may not be an early signal of apoptosis in neuronal cells, but only the phosphatidylserine-displaying apoptotic cells are phagocytosed by microglia. J. Neurochem. 1998;71:1854–1864. - PubMed
-
- Aroor AR, Baker RC. Ethanol inhibition of phagocytosis and superoxide anion production by microglia. Alcohol. 1998;15:277–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

