Synthesis of aromatic aza-metallapentalenes from metallabenzene via sequential ring contraction/annulation
- PMID: 25856747
- PMCID: PMC4391318
- DOI: 10.1038/srep09584
Synthesis of aromatic aza-metallapentalenes from metallabenzene via sequential ring contraction/annulation
Abstract
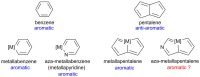
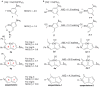
The concept of aromaticity has long played an important role in chemistry and continues to fascinate both experimentalists and theoreticians. Among the archetypal aromatic compounds, heteroaromatics are particularly attractive. Recently, substitution of a transition-metal fragment for a carbon atom in the anti-aromatic hydrocarbon pentalene has led to the new heteroaromatic osmapentalenes. However, construction of the aza-homolog of osmapentalenes cannot be accomplished by a similar synthetic manipulation. Here, we report the synthesis of aza-osmapentalenes by sequential ring contraction/annulation reactions of osmabenzenes via osmapentafulvenes. Nuclear magnetic resonance spectra, X-ray crystallographic analysis, and DFT calculations all suggest that these aza-osmapentalenes exhibit aromatic character. Thus, the stepwise transformation of metallabenzenes to metallapentafulvenes and then aza-metallapentalenes provides an efficient and facile synthetic route to these bicyclic heteroaromatics.
Figures




Similar articles
-
Unconventional Aromaticity in Organometallics: The Power of Transition Metals.Acc Chem Res. 2019 May 21;52(5):1449-1460. doi: 10.1021/acs.accounts.9b00092. Epub 2019 May 7. Acc Chem Res. 2019. PMID: 31062968
-
Metallabenzenes.Chem Rev. 2001 May;101(5):1205-27. doi: 10.1021/cr990337n. Chem Rev. 2001. PMID: 11710218
-
Recent Advances in Metallaaromatic Chemistry.Chemistry. 2018 Feb 9;24(9):2025-2038. doi: 10.1002/chem.201704888. Epub 2017 Dec 13. Chemistry. 2018. PMID: 29193396
-
Metallabenzenes and metallabenzenoids.Dalton Trans. 2006 Apr 21;(15):1821-7. doi: 10.1039/b517928a. Epub 2006 Mar 10. Dalton Trans. 2006. PMID: 16585967 Review.
-
Transition Metal-Catalyzed Intermolecular Cascade C-H Activation/Annulation Processes for the Synthesis of Polycycles.Chemistry. 2021 Jan 4;27(1):121-144. doi: 10.1002/chem.202002110. Epub 2020 Oct 15. Chemistry. 2021. PMID: 32530508 Review.
References
-
- Faraday M. On new compounds of carbon and hydrogen and on certain other products obtained during the decomposition of oil by heat. Philos. Trans. R. Soc. Lond. 115, 440–446 (1825).
-
- Kekulé F. A. Sur la constitution des substances aromatiques. Bull. Soc. Chim. 3, 98–111 (1865).
-
- Kekulé F. A. Note sur quelques produits de substitution de la benzine. Bull. Acad. R. Belg. 19, 551–563 (1865).
-
- Hückel E. Quantum-theoretical contributions to the benzene problem. I. The electron configuration of benzene and related compounds. Z. phys. 70, 204–286 (1931).
-
- Craig D. P. A novel type of aromaticity. Nature 181, 1052–1053 (1958).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

