Dominant Alcohol-Protein Interaction via Hydration-Enabled Enthalpy-Driven Binding Mechanism
- PMID: 25856773
- PMCID: PMC4465287
- DOI: 10.1021/acs.jpcb.5b00378
Dominant Alcohol-Protein Interaction via Hydration-Enabled Enthalpy-Driven Binding Mechanism
Abstract
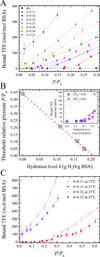
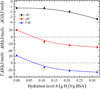
Water plays an important role in weak associations of small drug molecules with proteins. Intense focus has been on binding-induced structural changes in the water network surrounding protein binding sites, especially their contributions to binding thermodynamics. However, water is also tightly coupled to protein conformations and dynamics, and so far little is known about the influence of water-protein interactions on ligand binding. Alcohols are a type of low-affinity drugs, and it remains unclear how water affects alcohol-protein interactions. Here, we present alcohol adsorption isotherms under controlled protein hydration using in situ NMR detection. As functions of hydration level, Gibbs free energy, enthalpy, and entropy of binding were determined from the temperature dependence of isotherms. Two types of alcohol binding were found. The dominant type is low-affinity nonspecific binding, which is strongly dependent on temperature and the level of hydration. At low hydration levels, this nonspecific binding only occurs above a threshold of alcohol vapor pressure. An increased hydration level reduces this threshold, with it finally disappearing at a hydration level of h ≈ 0.2 (g water/g protein), gradually shifting alcohol binding from an entropy-driven to an enthalpy-driven process. Water at charged and polar groups on the protein surface was found to be particularly important in enabling this binding. Although further increase in hydration has smaller effects on the changes of binding enthalpy and entropy, it results in a significant negative change in Gibbs free energy due to unmatched enthalpy-entropy compensation. These results show the crucial role of water-protein interplay in alcohol binding.
Figures


References
-
- Breiten B, Lockett MR, Sherman W, Fujita S, Al-Sayah M, Lange H, Bowers CM, Heroux A, Krilov G, Whitesides GM. Water Networks Contribute to Enthalpy/Entropy Compensation in Protein–Ligand Binding. J. Am. Chem. Soc. 2013;135:15579–15584. - PubMed
-
- Li Z, Lazaridis T. Water at Biomolecular Binding Interfaces. Phys. Chem. Chem. Phys. 2007;9:573–581. - PubMed
-
- Levy Y, Onuchic JN. Water Mediation in Protein Folding and Molecular Recognition. Annu. Rev. Biophys. Biomol. Struct. 2006;35:389–415. - PubMed
-
- Chaplin M. Do We Underestimate the Importance of Water in Cell Biology? Nat. Rev. Mol. Cell Bio. 2006;7:861–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

