Acacetin inhibits expression of matrix metalloproteinases via a MAPK-dependent mechanism in fibroblast-like synoviocytes
- PMID: 25856795
- PMCID: PMC4549041
- DOI: 10.1111/jcmm.12564
Acacetin inhibits expression of matrix metalloproteinases via a MAPK-dependent mechanism in fibroblast-like synoviocytes
Abstract
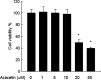
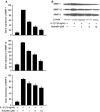
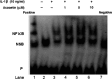
It is well known that rheumatoid arthritis (RA) is an autoimmune joint disease in which fibroblast-like synoviocytes (FLSs) play a pivotal role. In this study, we investigated the anti-arthritic properties of acacetin in FLSs. The expression of matrix metalloproteinase (MMP)-1, MMP-3 and MMP-13 were investigated by quantitative RT-PCR and western blot at gene and protein levels. At the same time, the phosphorylation of mitogen-activated protein kinases (MAPK) was investigated. The DNA-binding activity of NF-κB was investigated by electrophoretic mobility shift assay. We found that acacetin inhibits p38 and JNK phosphorylation and reduces MMP-1, MMP-3 and MMP-13 expression in interleukin-1β-induced FLSs. Our results suggest that acacetin has antiarthritic effects in FLSs. Thus, acacetin should be further studied for the treatment of arthritis.
Keywords: acacetin; fibroblast-like synoviocytes; interleukin-1β; matrix metalloproteinase; rheumatoid arthritis.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


Similar articles
-
Pyrrolidine dithiocarbamate, a NF-kappaB inhibitor, upregulates MMP-1 and MMP-13 in IL-1beta-stimulated rheumatoid arthritis fibroblast-like synoviocytes.Eur J Pharmacol. 2009 Jun 24;613(1-3):167-75. doi: 10.1016/j.ejphar.2009.04.026. Epub 2009 Apr 18. Eur J Pharmacol. 2009. PMID: 19379726
-
Taurine chloramine differentially inhibits matrix metalloproteinase 1 and 13 synthesis in interleukin-1beta stimulated fibroblast-like synoviocytes.Arthritis Res Ther. 2007;9(4):R80. doi: 10.1186/ar2279. Arthritis Res Ther. 2007. PMID: 17697361 Free PMC article.
-
Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1beta-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via down-regulation of NF-kappaB and p38.Rheumatology (Oxford). 2006 Jul;45(7):824-32. doi: 10.1093/rheumatology/kel026. Epub 2006 Jan 31. Rheumatology (Oxford). 2006. PMID: 16449361
-
SeMet inhibits IL-1β-induced rheumatoid fibroblast-like synoviocytes proliferation and the production of inflammatory mediators.Biol Trace Elem Res. 2013 Jun;153(1-3):437-45. doi: 10.1007/s12011-013-9696-6. Epub 2013 May 17. Biol Trace Elem Res. 2013. PMID: 23681674
-
The Role of Inflammation in the Pathogenesis of Osteoarthritis.Mediators Inflamm. 2020 Mar 3;2020:8293921. doi: 10.1155/2020/8293921. eCollection 2020. Mediators Inflamm. 2020. PMID: 32189997 Free PMC article. Review.
Cited by
-
Acacetin Suppresses IL-1β-Induced Expression of Matrix Metalloproteinases in Chondrocytes and Protects against Osteoarthritis in a Mouse Model by Inhibiting NF-κB Signaling Pathways.Biomed Res Int. 2020 Oct 26;2020:2328401. doi: 10.1155/2020/2328401. eCollection 2020. Biomed Res Int. 2020. PMID: 33195691 Free PMC article.
-
The potential mechanism of Huangqin for treatment of systemic lupus erythematosus based on network pharmacology, molecular docking and molecular dynamics simulation.PeerJ. 2025 Jun 26;13:e19536. doi: 10.7717/peerj.19536. eCollection 2025. PeerJ. 2025. PMID: 40585331 Free PMC article.
-
Acacetin Protects Mice from Staphylococcus aureus Bloodstream Infection by Inhibiting the Activity of Sortase A.Molecules. 2016 Sep 26;21(10):1285. doi: 10.3390/molecules21101285. Molecules. 2016. PMID: 27681715 Free PMC article.
-
Unraveling the Therapeutic Mechanism of Saussurea involucrata against Rheumatoid Arthritis: A Network Pharmacology and Molecular Modeling-Based Investigation.Nutrients. 2023 Oct 9;15(19):4294. doi: 10.3390/nu15194294. Nutrients. 2023. PMID: 37836578 Free PMC article.
-
Inhibitory Effect of Lygodium Root on the Cytochrome P450 3A Enzyme in vitro and in vivo.Drug Des Devel Ther. 2020 May 19;14:1909-1919. doi: 10.2147/DDDT.S249308. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32546958 Free PMC article.
References
-
- Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–70. - PubMed
-
- Lee YR, Jin GH, Lee SM, et al. Inhibition of TNF-α-mediated inflammatory responses by a benzodioxolylacetylamino-linked benzothiazole analog in human fibroblast-like synoviocytes. Biochem Biophys Res Commun. 2011;4:625–9. - PubMed
-
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

