The role of photodynamic therapy in overcoming cancer drug resistance
- PMID: 25856800
- PMCID: PMC4520758
- DOI: 10.1039/c4pp00495g
The role of photodynamic therapy in overcoming cancer drug resistance
Abstract
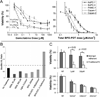
Many modalities of cancer therapy induce mechanisms of treatment resistance and escape pathways during chronic treatments, including photodynamic therapy (PDT). It is conceivable that resistance induced by one treatment might be overcome by another treatment. Emerging evidence suggests that the unique mechanisms of tumor cell and microenvironment damage produced by PDT could be utilized to overcome cancer drug resistance, to mitigate the compensatory induction of survival pathways and even to re-sensitize resistant cells to standard therapies. Approaches that capture the unique features of PDT, therefore, offer promising factors for increasing the efficacy of a broad range of therapeutic modalities. Here, we highlight key preclinical findings utilizing PDT to overcome classical drug resistance or escape pathways and thus enhance the efficacy of many pharmaceuticals, possibly explaining the clinical observations of the PDT response to otherwise treatment-resistant diseases. With the development of nanotechnology, it is possible that light activation may be used not only to damage and sensitize tumors but also to enable controlled drug release to inhibit escape pathways that may lead to resistance or cell proliferation.
Figures



References
-
- Milano MT, et al. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:1147–1155. - PubMed
-
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. - PubMed
-
- Epithelial-mesenchymal transitions in development and disease. 2009;139:871–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

