α1 -Adrenoceptor activation of PKC-ε causes heterologous desensitization of thromboxane receptors in the aorta of spontaneously hypertensive rats
- PMID: 25857252
- PMCID: PMC4507169
- DOI: 10.1111/bph.13157
α1 -Adrenoceptor activation of PKC-ε causes heterologous desensitization of thromboxane receptors in the aorta of spontaneously hypertensive rats
Abstract
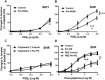
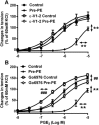
Background and purpose: In the aorta of adult spontaneously hypertensive (SHR), but not in that of normotensive Wistar-Kyoto (WKY), rats, previous exposure to phenylephrine inhibits subsequent contractions to PGE2 . The present experiments were designed to examine the mechanism(s) underlying this inhibition.
Experimental approach: Isometric tension was measured in isolated rings of SHR and WKY aortae. Gene expression and protein presence were measured by quantitative real-time PCR and Western blotting respectively.
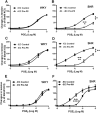
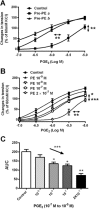
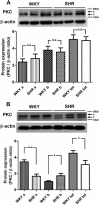
Key results: In aorta of 18 weeks SHR, but not age-matched WKY, pre-exposure to phenylephrine inhibited subsequent contractions to PGE2 that were mediated by thromboxane prostanoid (TP) receptors. This inhibition was not observed in preparations of pre-hypertensive 5-week-old SHR, and was significantly larger in those of 36- than 18-week-old SHR. Pre-exposure to the PKC activator, phorbol 12,13-dibutyrate, also inhibited subsequent contractions to PGE2 in SHR aortae. The selective inhibitor of PKC-ε, ε-V1-2, abolished the desensitization caused by pre-exposure to phenylephrine. Two molecular PKC bands were detected and their relative intensities differed in 36-week-old WKY and SHR vascular smooth muscle. The mRNA expressions of PKC-α, PKC-ε, PK-N2 and PKC-ζ and of G protein-coupled kinase (GRK)-2, GRK4 and β-arrestin2 were higher in SHR than WKY aortae.
Conclusions and implications: These experiments suggest that in the SHR but not the WKY aorta, α1 -adrenoceptor activation desensitizes TP receptors through activation of PKC-ε. This heterologous desensitization is a consequence of the chronic exposure to high arterial pressure.
© 2015 The British Pharmacological Society.
Figures



References
-
- Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J Biochem. 2002;132:847–852. - PubMed
-
- Avendano MS, Lucas E, Jurado-Pueyo M, Martinez-Revelles S, Vila-Bedmar R, Mayor F, Jr, et al. Increased nitric oxide bioavailability in adult GRK2 hemizygous mice protects against angiotensin II-induced hypertension. Hypertension. 2014;63:369–375. - PubMed
-
- Baretella O, Xu A, Vanhoutte PM. Acidosis prevents and alkalosis augments endothelium-dependent contractions in mouse arteries. Pflugers Arch. 2014;466:295–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

