A proton current associated with sour taste: distribution and functional properties
- PMID: 25857556
- PMCID: PMC4763920
- DOI: 10.1096/fj.14-265694
A proton current associated with sour taste: distribution and functional properties
Abstract
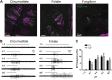
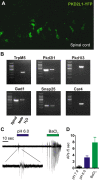
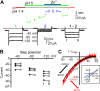
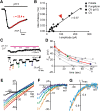
Sour taste is detected by taste receptor cells that respond to acids through yet poorly understood mechanisms. The cells that detect sour express the protein PKD2L1, which is not the sour receptor but nonetheless serves as a useful marker for sour cells. By use of mice in which the PKD2L1 promoter drives expression of yellow fluorescent protein, we previously reported that sour taste cells from circumvallate papillae in the posterior tongue express a proton current. To establish a correlation between this current and sour transduction, we examined its distribution by patch-clamp recording. We find that the current is present in PKD2L1-expressing taste cells from mouse circumvallate, foliate, and fungiform papillae but not in a variety of other cells, including spinal cord neurons that express PKD2L1. We describe biophysical properties of the current, including pH-dependent Zn(2+) inhibition, lack of voltage-dependent gating, and activation at modest pH values (6.5) that elicit action potentials in isolated cells. Consistent with a channel that is constitutively open, the cytosol of sour taste cells is acidified. These data define a functional signature for the taste cell proton current and indicate that its expression is mostly restricted to the subset of taste cells that detect sour.
Keywords: PKD2L1; acid; ion channel; pH; sensory transduction.
© FASEB.
Figures





Similar articles
-
A proton current drives action potentials in genetically identified sour taste cells.Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22320-5. doi: 10.1073/pnas.1013664107. Epub 2010 Nov 23. Proc Natl Acad Sci U S A. 2010. PMID: 21098668 Free PMC article.
-
The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction.Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):E229-38. doi: 10.1073/pnas.1514282112. Epub 2015 Dec 1. Proc Natl Acad Sci U S A. 2016. PMID: 26627720 Free PMC article.
-
Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells.J Biol Chem. 2010 Jun 4;285(23):17277-81. doi: 10.1074/jbc.C110.132944. Epub 2010 Apr 20. J Biol Chem. 2010. PMID: 20406802 Free PMC article.
-
Identification of sour-taste receptor genes.Anat Sci Int. 2003 Dec;78(4):205-10. doi: 10.1046/j.0022-7722.2003.00062.x. Anat Sci Int. 2003. PMID: 14686475 Review.
-
Taste receptors in the gastrointestinal tract III. Salty and sour taste: sensing of sodium and protons by the tongue.Am J Physiol Gastrointest Liver Physiol. 2006 Dec;291(6):G1005-10. doi: 10.1152/ajpgi.00235.2006. Epub 2006 Jun 29. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16809639 Review.
Cited by
-
Zinc activation of OTOP proton channels identifies structural elements of the gating apparatus.Elife. 2023 Apr 13;12:e85317. doi: 10.7554/eLife.85317. Elife. 2023. PMID: 37053086 Free PMC article.
-
Ionotropic Receptors Mediate Drosophila Oviposition Preference through Sour Gustatory Receptor Neurons.Curr Biol. 2017 Sep 25;27(18):2741-2750.e4. doi: 10.1016/j.cub.2017.08.003. Epub 2017 Sep 7. Curr Biol. 2017. PMID: 28889974 Free PMC article.
-
Preferential allosteric modulation of Otop1 channels by small molecule compounds.Commun Biol. 2025 Feb 26;8(1):314. doi: 10.1038/s42003-025-07775-9. Commun Biol. 2025. PMID: 40011703 Free PMC article.
-
The cellular mechanism for water detection in the mammalian taste system.Nat Neurosci. 2017 Jul;20(7):927-933. doi: 10.1038/nn.4575. Epub 2017 May 29. Nat Neurosci. 2017. PMID: 28553944
-
Progress and renewal in gustation: new insights into taste bud development.Development. 2015 Nov 1;142(21):3620-9. doi: 10.1242/dev.120394. Development. 2015. PMID: 26534983 Free PMC article. Review.
References
-
- Gilbertson T. A., Roper S. D., Kinnamon S. C. (1993) Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: enhancement by vasopressin and cAMP. Neuron 10, 931–942 - PubMed
-
- Ugawa S., Minami Y., Guo W., Saishin Y., Takatsuji K., Yamamoto T., Tohyama M., Shimada S. (1998) Receptor that leaves a sour taste in the mouth. Nature 395, 555–556 - PubMed
-
- Stevens D. R., Seifert R., Bufe B., Müller F., Kremmer E., Gauss R., Meyerhof W., Kaupp U. B., Lindemann B. (2001) Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature 413, 631–635 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

