Mechanisms of bone marrow-derived cell therapy in ischemic cardiomyopathy with left ventricular assist device bridge to transplant
- PMID: 25857908
- PMCID: PMC4771180
- DOI: 10.1016/j.jacc.2015.01.042
Mechanisms of bone marrow-derived cell therapy in ischemic cardiomyopathy with left ventricular assist device bridge to transplant
Abstract
Background: Clinical trials report improvements in function and perfusion with direct injection of bone marrow cells into the hearts of patients with ischemic cardiomyopathy. Preclinical data suggest these cells improve vascular density, which would be expected to decrease fibrosis and inflammation.
Objectives: The goal of this study was to test the hypothesis that bone marrow stem cells (CD34+) will improve histological measurements of vascularity, fibrosis, and inflammation in human subjects undergoing left ventricular assist device (LVAD) placement as a bridge to cardiac transplantation.
Methods: Subjects with ischemic cardiomyopathy who were scheduled for placement of an LVAD as a bridge to transplantation underwent bone marrow aspiration the day before surgery; the bone marrow was processed into cell fractions (bone marrow mononuclear cells, CD34+, and CD34-). At LVAD implantation, all fractions and a saline control were injected epicardially into predetermined areas and each injection site marked. At the time of transplantation, injected areas were collected. Data were analyzed by paired Student t test comparing the effect of cell fractions injected within each subject.
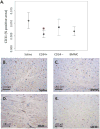
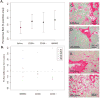
Results: Six subjects completed the study. There were no statistically significant differences in complications with the procedure versus control subjects. Histological analysis indicated that myocardium injected with CD34+ cells had decreased density of endothelial cells compared to saline-injected myocardium. There were no significant differences in fibrosis or inflammation between groups; however, density of activated fibroblasts was decreased in both CD34+ and CD34- injected areas.
Conclusions: Tissue analysis does not support the hypothesis that bone marrow-derived CD34+ cells promote increased vascular tissue in humans with ischemic cardiomyopathy via direct injection.
Keywords: angiogenesis; cell therapy; heart failure; ischemia.
Copyright © 2015 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Of mice and men: the best laid scheme?J Am Coll Cardiol. 2015 Apr 14;65(14):1435-7. doi: 10.1016/j.jacc.2015.02.035. J Am Coll Cardiol. 2015. PMID: 25857909 No abstract available.
References
-
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. - PubMed
-
- Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. - PubMed
-
- Thompson RB, van den Bos EJ, Davis BH, et al. Intracardiac transplantation of a mixed population of bone marrow cells improves both regional systolic contractility and diastolic relaxation. J Heart Lung Transplant. 2005;24:205–14. - PubMed
-
- Tse HF, Siu CW, Zhu SG, et al. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur J Heart Fail. 2007;9:747–53. - PubMed
-
- Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

