Use of mesenchymal stem cells for therapy of cardiac disease
- PMID: 25858066
- PMCID: PMC4429294
- DOI: 10.1161/CIRCRESAHA.116.303614
Use of mesenchymal stem cells for therapy of cardiac disease
Abstract
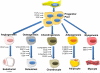
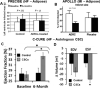
Despite substantial clinical advances over the past 65 years, cardiovascular disease remains the leading cause of death in America. The past 15 years has witnessed major basic and translational interest in the use of stem and precursor cells as a therapeutic agent for chronically injured organs. Among the cell types under investigation, adult mesenchymal stem cells are widely studied, and in early stage, clinical studies show promise for repair and regeneration of cardiac tissues. The ability of mesenchymal stem cells to differentiate into mesoderm- and nonmesoderm-derived tissues, their immunomodulatory effects, their availability, and their key role in maintaining and replenishing endogenous stem cell niches have rendered them one of the most heavily investigated and clinically tested type of stem cell. Accumulating data from preclinical and early phase clinical trials document their safety when delivered as either autologous or allogeneic forms in a range of cardiovascular diseases, but also importantly define parameters of clinical efficacy that justify further investigation in larger clinical trials. Here, we review the biology of mesenchymal stem cells, their interaction with endogenous molecular and cellular pathways, and their modulation of immune responses. Additionally, we discuss factors that enhance their proliferative and regenerative ability and factors that may hinder their effectiveness in the clinical setting.
Keywords: cell differentiation; mesenchymal stem cell; regeneration; stem cells.
© 2015 American Heart Association, Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

