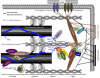
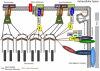
Mechanotransduction in cardiac hypertrophy and failure
- PMID: 25858069
- PMCID: PMC4394185
- DOI: 10.1161/CIRCRESAHA.116.304937
Mechanotransduction in cardiac hypertrophy and failure
Abstract
Cardiac muscle cells have an intrinsic ability to sense and respond to mechanical load through a process known as mechanotransduction. In the heart, this process involves the conversion of mechanical stimuli into biochemical events that induce changes in myocardial structure and function. Mechanotransduction and its downstream effects function initially as adaptive responses that serve as compensatory mechanisms during adaptation to the initial load. However, under prolonged and abnormal loading conditions, the remodeling processes can become maladaptive, leading to altered physiological function and the development of pathological cardiac hypertrophy and heart failure. Although the mechanisms underlying mechanotransduction are far from being fully elucidated, human and mouse genetic studies have highlighted various cytoskeletal and sarcolemmal structures in cardiac myocytes as the likely candidates for load transducers, based on their link to signaling molecules and architectural components important in disease pathogenesis. In this review, we summarize recent developments that have uncovered specific protein complexes linked to mechanotransduction and mechanotransmission within the sarcomere, the intercalated disc, and at the sarcolemma. The protein structures acting as mechanotransducers are the first step in the process that drives physiological and pathological cardiac hypertrophy and remodeling, as well as the transition to heart failure, and may provide better insights into mechanisms driving mechanotransduction-based diseases.
Keywords: cytoskeleton; heart; heart failure; intercellular junctions; mechanotransduction, cellular; myocardium; sarcolemma; sarcomeres.
© 2015 American Heart Association, Inc.
Figures