Hepcidin regulation in prostate and its disruption in prostate cancer
- PMID: 25858146
- PMCID: PMC4454355
- DOI: 10.1158/0008-5472.CAN-14-2465
Hepcidin regulation in prostate and its disruption in prostate cancer
Abstract
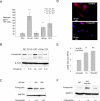
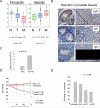
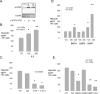
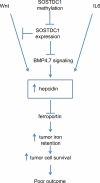
Hepcidin is a circulating peptide hormone made by the liver that is a central regulator of systemic iron uptake and recycling. Here, we report that prostate epithelial cells also synthesize hepcidin, and that synthesis and secretion of hepcidin are markedly increased in prostate cancer cells and tissue. Prostatic hepcidin functions as an autocrine hormone, decreasing cell surface ferroportin, an iron exporter, increasing intracellular iron retention, and promoting prostate cancer cell survival. Synthesis of hepcidin in prostate cancer is controlled by a unique intersection of pathways that involves BMP4/7, IL6, Wnt, and the dual BMP and Wnt antagonist, SOSTDC1. Epigenetic silencing of SOSTDC1 through methylation is increased in prostate cancer and is associated with accelerated disease progression in patients with prostate cancer. These results establish a new connection between iron metabolism and prostate cancer, and suggest that prostatic dysregulation of hepcidin contributes to prostate cancer growth and progression.
©2015 American Association for Cancer Research.
Figures



References
-
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (New York, NY) 2004;306:2090–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

