Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial
- PMID: 25858265
- PMCID: PMC4391970
- DOI: 10.1136/bmj.h1389
Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial
Abstract
Objective: To evaluate whether a disease activity guided strategy of dose reduction of two tumour necrosis factor (TNF) inhibitors, adalimumab or etanercept, is non-inferior in maintaining disease control in patients with rheumatoid arthritis compared with usual care.
Design: Randomised controlled, open label, non-inferiority strategy trial.
Setting: Two rheumatology outpatient clinics in the Netherlands, from December 2011 to May 2014.
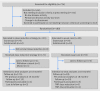
Participants: 180 patients with rheumatoid arthritis and low disease activity using adalimumab or etanercept; 121 allocated to the dose reduction strategy, 59 to usual care.
Interventions: Disease activity guided dose reduction (advice to stepwise increase the injection interval every three months, until flare of disease activity or discontinuation) or usual care (no dose reduction advice). Flare was defined as increase in DAS28-CRP (a composite score measuring disease activity) greater than 1.2, or increase greater than 0.6 and current score of at least 3.2. In the case of flare, TNF inhibitor use was restarted or escalated.
Main outcome measures: Difference in proportions of patients with major flare (DAS28-CRP based flare longer than three months) between the two groups at 18 months, compared against a non-inferiority margin of 20%. Secondary outcomes included TNF inhibitor use at study end, functioning, quality of life, radiographic progression, and adverse events.
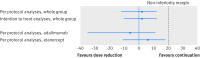
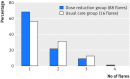
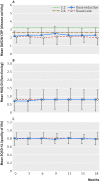
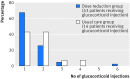
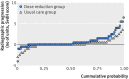
Results: Dose reduction of adalimumab or etanercept was non-inferior to usual care (proportion of patients with major flare at 18 months, 12% v 10%; difference 2%, 95% confidence interval -12% to 12%). In the dose reduction group, TNF inhibitor use could successfully be stopped in 20% (95% confidence interval 13% to 28%), the injection interval successfully increased in 43% (34% to 53%), but no dose reduction was possible in 37% (28% to 46%). Functional status, quality of life, relevant radiographic progression, and adverse events did not differ between the groups, although short lived flares (73% v 27%) and minimal radiographic progression (32% v 15%) were more frequent in dose reduction than usual care.
Conclusions: A disease activity guided, dose reduction strategy of adalimumab or etanercept to treat rheumatoid arthritis is non-inferior to usual care with regard to major flaring, while resulting in the successful dose reduction or stopping in two thirds of patients.Trial registration Dutch trial register (www.trialregister.nl), NTR 3216.
© van Herwaarden et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Disease activity-guided TNF inhibitor dose reduction was noninferior to continuing TNF inhibitors for RA flares.Ann Intern Med. 2015 Sep 15;163(6):JC9. doi: 10.7326/ACPJC-2015-163-6-009. Ann Intern Med. 2015. PMID: 26370036 No abstract available.
Similar articles
-
Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity.Cochrane Database Syst Rev. 2019 May 24;5(5):CD010455. doi: 10.1002/14651858.CD010455.pub3. Cochrane Database Syst Rev. 2019. PMID: 31125448 Free PMC article.
-
A multi-biomarker score measuring disease activity in rheumatoid arthritis patients tapering adalimumab or etanercept: predictive value for clinical and radiographic outcomes.Rheumatology (Oxford). 2017 Jun 1;56(6):973-980. doi: 10.1093/rheumatology/kex003. Rheumatology (Oxford). 2017. PMID: 28339738 Clinical Trial.
-
Tumour necrosis factor inhibitors versus combination intensive therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: TACIT non-inferiority randomised controlled trial.BMJ. 2015 Mar 13;350:h1046. doi: 10.1136/bmj.h1046. BMJ. 2015. PMID: 25769495 Free PMC article. Clinical Trial.
-
Long-term outcomes after disease activity-guided dose reduction of TNF inhibition in rheumatoid arthritis: 3-year data of the DRESS study - a randomised controlled pragmatic non-inferiority strategy trial.Ann Rheum Dis. 2017 Oct;76(10):1716-1722. doi: 10.1136/annrheumdis-2017-211169. Epub 2017 Jun 12. Ann Rheum Dis. 2017. PMID: 28606961 Clinical Trial.
-
[TNF inhibitors for treatment of rheumatoid arthritis].Nihon Naika Gakkai Zasshi. 2008 Oct 10;97(10):2405-12. doi: 10.2169/naika.97.2405. Nihon Naika Gakkai Zasshi. 2008. PMID: 19149036 Review. Japanese. No abstract available.
Cited by
-
Cost-utility analysis of de-escalating biological disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis.PLoS One. 2020 Jan 2;15(1):e0226754. doi: 10.1371/journal.pone.0226754. eCollection 2020. PLoS One. 2020. PMID: 31895926 Free PMC article.
-
What causes a small increase in radiographic progression in rheumatoid arthritis patients tapering TNF inhibitors?RMD Open. 2017 Mar 20;3(1):e000327. doi: 10.1136/rmdopen-2016-000327. eCollection 2017. RMD Open. 2017. PMID: 28405469 Free PMC article.
-
A Systematic Review to Identify the Effects of Biologics in the Feet of Patients with Rheumatoid Arthritis.Medicina (Kaunas). 2020 Dec 29;57(1):23. doi: 10.3390/medicina57010023. Medicina (Kaunas). 2020. PMID: 33383830 Free PMC article.
-
Non-adherence in non-inferiority trials: pitfalls and recommendations.BMJ. 2020 Jul 1;370:m2215. doi: 10.1136/bmj.m2215. BMJ. 2020. PMID: 32611541 Free PMC article.
-
[Rheumatoid arthritis].Z Rheumatol. 2017 Feb;76(1):8-14. doi: 10.1007/s00393-016-0251-7. Z Rheumatol. 2017. PMID: 28058499 Review. German.
References
-
- Graudal N, Jürgens G. Similar effects of disease-modifying antirheumatic drugs, glucocorticoids, and biologic agents on radiographic progression in rheumatoid arthritis: meta-analysis of 70 randomized placebo-controlled or drug-controlled studies, including 112 comparisons. Arthritis Rheum 2010;62:2852-63. - PubMed
-
- Huggett B. Public biotech 2012—the numbers. Nat Biotechnol 2013;31:697-703. - PubMed
-
- King S. FirstWord Lists —Pharma’s 50 biggest selling drugs: AbbVie’s Humira joins the $10 billion club. 7 March 2014. www.firstwordpharma.com/node/1194000#axzz3UYi8L3BI.
-
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275-85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
