AMPK Suppresses Vascular Inflammation In Vivo by Inhibiting Signal Transducer and Activator of Transcription-1
- PMID: 25858560
- PMCID: PMC4657575
- DOI: 10.2337/db15-0107
AMPK Suppresses Vascular Inflammation In Vivo by Inhibiting Signal Transducer and Activator of Transcription-1
Expression of concern in
-
Expression of Concern. Chaoyong He, Hongliang Li, Benoit Viollet, Ming-Hui Zou, and Zhonglin Xie. AMPK Suppresses Vascular Inflammation In Vivo by Inhibiting Signal Transducer and Activator of Transcription-1. Diabetes 2015;64:4285-4297. DOI: 10.2337/db15-0107. PMID: 25858560. PMCID: PMC4657575.Diabetes. 2023 Jul 1;72(7):1038. doi: 10.2337/db23-ec07c. Diabetes. 2023. PMID: 37184353 Free PMC article. No abstract available.
Abstract
Activation of AMPK suppresses inflammation, but the underlying mechanisms remain poorly understood. This study was designed to characterize the molecular mechanisms by which AMPK suppresses vascular inflammation. In cultured human aortic smooth muscle cells, pharmacologic or genetic activation of AMPK inhibited the signal transducer and activator of transcription-1 (STAT1), while inhibition of AMPK had opposite effects. Deletion of AMPKα1 or AMPKα2 resulted in activation of STAT1 and in increases in proinflammatory mediators, both of which were attenuated by administration of STAT1 small interfering RNA or fludarabine, a selective STAT1 inhibitor. Moreover, AMPK activation attenuated the proinflammatory actions induced by STAT1 activators such as interferon-γ and angiotensin II (AngII). Mechanistically, we found that AMPK activation increased, whereas AMPK inhibition decreased, the levels of mitogen-activated protein kinase phosphatase-1 (MKP-1), an inducible nuclear phosphatase, by regulating proteasome-dependent degradation of MKP-1. Gene silencing of MKP-1 increased STAT1 phosphorylation and prevented 5-aminoimidazole-4-carboxyamide ribonucleoside-reduced STAT1 phosphorylation. Finally, we found that infusion of AngII caused a more severe inflammatory response in AMPKα2 knockout mouse aortas, all of which were suppressed by chronic administration of fludarabine. We conclude that AMPK activation suppresses STAT1 signaling and inhibits vascular inflammation through the upregulation of MKP-1.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

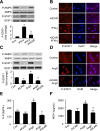


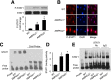



Similar articles
-
Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling.Biochem Pharmacol. 2016 Feb 1;101:54-70. doi: 10.1016/j.bcp.2015.11.026. Epub 2015 Nov 28. Biochem Pharmacol. 2016. PMID: 26643070 Free PMC article.
-
Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1.Endocrinology. 2008 Aug;149(8):4086-94. doi: 10.1210/en.2007-1639. Epub 2008 May 8. Endocrinology. 2008. PMID: 18467446 Free PMC article.
-
Inhibition of the AMP-activated protein kinase-α2 accentuates agonist-induced vascular smooth muscle contraction and high blood pressure in mice.Hypertension. 2011 May;57(5):1010-7. doi: 10.1161/HYPERTENSIONAHA.110.168906. Epub 2011 Apr 4. Hypertension. 2011. PMID: 21464390 Free PMC article.
-
Adenosine monophosphate-activated protein kinase-α2 deficiency promotes vascular smooth muscle cell migration via S-phase kinase-associated protein 2 upregulation and E-cadherin downregulation.Arterioscler Thromb Vasc Biol. 2013 Dec;33(12):2800-9. doi: 10.1161/ATVBAHA.113.301869. Epub 2013 Oct 10. Arterioscler Thromb Vasc Biol. 2013. Retraction in: Arterioscler Thromb Vasc Biol. 2022 Nov;42(11):e290. doi: 10.1161/ATV.0000000000000158. PMID: 24115035 Free PMC article. Retracted.
-
Role of AMP-activated protein kinase α1 in 17α-ethinylestradiol-induced cholestasis in rats.Arch Toxicol. 2017 Jan;91(1):481-494. doi: 10.1007/s00204-016-1697-8. Epub 2016 Apr 18. Arch Toxicol. 2017. PMID: 27090119 Free PMC article.
Cited by
-
Exercise mimetics and JAK inhibition attenuate IFN-γ-induced wasting in engineered human skeletal muscle.Sci Adv. 2021 Jan 22;7(4):eabd9502. doi: 10.1126/sciadv.abd9502. Print 2021 Jan. Sci Adv. 2021. PMID: 33523949 Free PMC article.
-
Direct Cardiac Actions of Sodium Glucose Cotransporter 2 Inhibitors Target Pathogenic Mechanisms Underlying Heart Failure in Diabetic Patients.Front Physiol. 2018 Nov 21;9:1575. doi: 10.3389/fphys.2018.01575. eCollection 2018. Front Physiol. 2018. PMID: 30519189 Free PMC article. Review.
-
Suppressor of Cytokine Signaling-1/STAT1 Regulates Renal Inflammation in Mesangial Proliferative Glomerulonephritis Models.Front Immunol. 2018 Aug 30;9:1982. doi: 10.3389/fimmu.2018.01982. eCollection 2018. Front Immunol. 2018. PMID: 30214448 Free PMC article.
-
Salicylates Ameliorate Intestinal Inflammation by Activating Macrophage AMPK.Inflamm Bowel Dis. 2021 May 17;27(6):914-926. doi: 10.1093/ibd/izaa305. Inflamm Bowel Dis. 2021. PMID: 33252129 Free PMC article.
-
Novel Anti-inflammatory Effects of Canagliflozin Involving Hexokinase II in Lipopolysaccharide-Stimulated Human Coronary Artery Endothelial Cells.Cardiovasc Drugs Ther. 2021 Dec;35(6):1083-1094. doi: 10.1007/s10557-020-07083-w. Epub 2020 Oct 13. Cardiovasc Drugs Ther. 2021. PMID: 33048256 Free PMC article.
References
-
- Steinberg GR, Schertzer JD. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunol Cell Biol 2014;92:340–345 - PubMed
-
- Ihle JN. STATs: signal transducers and activators of transcription. Cell 1996;84:331–334 - PubMed
-
- Recio C, Oguiza A, Lazaro I, Mallavia B, Egido J, Gomez-Guerrero C. Suppressor of cytokine signaling 1-derived peptide inhibits Janus kinase/signal transducers and activators of transcription pathway and improves inflammation and atherosclerosis in diabetic mice. Arterioscler Thromb Vasc Biol 2014;34:1953–1960 - PubMed
-
- Manea A, Tanase LI, Raicu M, Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 2010;30:105–112 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL074399/HL/NHLBI NIH HHS/United States
- R01 HL079584/HL/NHLBI NIH HHS/United States
- HL-080499/HL/NHLBI NIH HHS/United States
- HL-074399/HL/NHLBI NIH HHS/United States
- R43 HL110448/HL/NHLBI NIH HHS/United States
- R01 HL096032/HL/NHLBI NIH HHS/United States
- HL-08920/HL/NHLBI NIH HHS/United States
- HL-105157/HL/NHLBI NIH HHS/United States
- HL-096032/HL/NHLBI NIH HHS/United States
- HL-079584/HL/NHLBI NIH HHS/United States
- R01 HL105157/HL/NHLBI NIH HHS/United States
- R44 HL110448/HL/NHLBI NIH HHS/United States
- HL-110448/HL/NHLBI NIH HHS/United States
- P20 RR024215/RR/NCRR NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

