Microbial community composition shapes enzyme patterns in topsoil and subsoil horizons along a latitudinal transect in Western Siberia
- PMID: 25859057
- PMCID: PMC4381299
- DOI: 10.1016/j.soilbio.2015.01.016
Microbial community composition shapes enzyme patterns in topsoil and subsoil horizons along a latitudinal transect in Western Siberia
Abstract
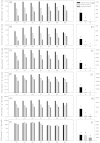
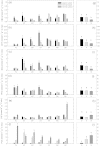
Soil horizons below 30 cm depth contain about 60% of the organic carbon stored in soils. Although insight into the physical and chemical stabilization of soil organic matter (SOM) and into microbial community composition in these horizons is being gained, information on microbial functions of subsoil microbial communities and on associated microbially-mediated processes remains sparse. To identify possible controls on enzyme patterns, we correlated enzyme patterns with biotic and abiotic soil parameters, as well as with microbial community composition, estimated using phospholipid fatty acid profiles. Enzyme patterns (i.e. distance-matrixes calculated from these enzyme activities) were calculated from the activities of six extracellular enzymes (cellobiohydrolase, leucine-amino-peptidase, N-acetylglucosaminidase, chitotriosidase, phosphatase and phenoloxidase), which had been measured in soil samples from organic topsoil horizons, mineral topsoil horizons, and mineral subsoil horizons from seven ecosystems along a 1500 km latitudinal transect in Western Siberia. We found that hydrolytic enzyme activities decreased rapidly with depth, whereas oxidative enzyme activities in mineral horizons were as high as, or higher than in organic topsoil horizons. Enzyme patterns varied more strongly between ecosystems in mineral subsoil horizons than in organic topsoils. The enzyme patterns in topsoil horizons were correlated with SOM content (i.e., C and N content) and microbial community composition. In contrast, the enzyme patterns in mineral subsoil horizons were related to water content, soil pH and microbial community composition. The lack of correlation between enzyme patterns and SOM quantity in the mineral subsoils suggests that SOM chemistry, spatial separation or physical stabilization of SOM rather than SOM content might determine substrate availability for enzymatic breakdown. The correlation of microbial community composition and enzyme patterns in all horizons, suggests that microbial community composition shapes enzyme patterns and might act as a modifier for the usual dependency of decomposition rates on SOM content or C/N ratios.
Keywords: Boreal forests; Extracellular enzymes; PLFA; Permafrost; Steppe; Tundra.
Figures



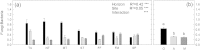
References
-
- Archipov S.A., Vdovon V.V., Mizerov B.V., Nikolaev V.A. Moskva Nauka; 1970. Zapadno-Sibirskaja ravnina. 278 p. (in Russian)
-
- Bach C.E., Warnock D.D., Van Horn D.J., Weintraub M.N., Sinsabaugh R.L., Allison S.D., German D.P. Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: effect of assay conditions and soil type. Soil Biology & Biochemistry. 2013;67:183–191.
-
- Brockett B.F., Prescott C.E., Grayston S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biology & Biochemistry. 2012;44:9–20.
-
- Brookes P.C., Landman A., Pruden G., Jenkinson D.S. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology & Biochemistry. 1985;17:837–842.
-
- Colman B.P., Schimel J.P. Drivers of microbial respiration and net N mineralization at the continental scale. Soil Biology & Biochemistry. 2013;60:65–76.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
