The role of carbohydrates in infection strategies of enteric pathogens
- PMID: 25859152
- PMCID: PMC4361345
- DOI: 10.2149/tmh.2014-25
The role of carbohydrates in infection strategies of enteric pathogens
Abstract
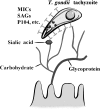
Enteric pathogens cause considerable public health concerns worldwide including tropical regions. Here, we review the roles of carbohydrates in the infection strategies of various enteric pathogens including viruses, bacteria and protozoa, which infect the epithelial lining of the human and animal intestine. At host cell entry, enteric viruses, including norovirus, recognize mainly histo-blood group antigens. At the initial step of bacterial infections, carbohydrates also function as receptors for attachment. Here, we describe the function of carbohydrates in infection by Salmonella enterica and several bacterial species that produce a variety of fimbrial adhesions. During invasion by enteropathogenic protozoa, apicomplexan parasites utilize sialic acids or sulfated glycans. Carbohydrates serve as receptors for infection by these microbes; however, their usage of carbohydrates varies depending on the microbe. On the surface of the mucosal tissues of the gastrointestinal tract, various carbohydrate moieties are present and play a crucial role in infection, representing the site of infection or route of access for most microbes. During the infection and/or invasion process of the microbes, carbohydrates function as receptors for various microbes, but they can also function as a barrier to infection. One approach to develop effective prophylactic and therapeutic antimicrobial agents is to modify the drug structure. Another approach is to modify the mode of inhibition of infection depending on the individual pathogen by using and mimicking the interactions with carbohydrates. In addition, similarities in mode of infection may also be utilized. Our findings will be useful in the development of new drugs for the treatment of enteric pathogens.
Keywords: bacteria; carbohydrate; enteric pathogen; infection; protozoa; virus.
Figures
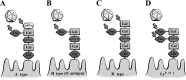


References
-
- Spear PG, Shieh MT, Herold BC, et al. . Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv Exp Med Biol 1992; 313: 341–353. - PubMed
-
- Lindesmith L, Moe C, Marionneau S, et al. . Human susceptibility and resistance to Norwalk virus infection. Nat Med 2003; 9: 548–553. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
