Aging, Alzheimer's, and APOE genotype influence the expression and neuronal distribution patterns of microtubule motor protein dynactin-P50
- PMID: 25859183
- PMCID: PMC4373372
- DOI: 10.3389/fncel.2015.00103
Aging, Alzheimer's, and APOE genotype influence the expression and neuronal distribution patterns of microtubule motor protein dynactin-P50
Abstract
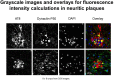
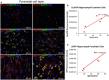
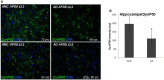
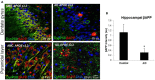
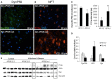
Reports from neural cell cultures and experimental animal studies provide evidence of age- and disease-related changes in retrograde transport of spent or misfolded proteins destined for degradation or recycling. However, few studies address these issues in human brain from those who either age without dementia and overt neuropathology, or succumb to Alzheimer's; especially as such propensity may be influenced by APOE genotype. We studied the expression and distribution of the dynein subunit dynactin-P50, the β amyloid precursor protein (βAPP), and hyperphosphorylated tau (P-tau) in tissues and tissue sections of brains from non-demented, neuropathology-free patients and from Alzheimer patients, with either APOE ε3,3 or APOE ε4,4. We found that advanced age in patients without dementia or neuropathological change was associated with coordinated increases in dynactin-P50 and βAPP in neurons in pyramidal layers of the hippocampus. In contrast, in Alzheimer's, βAPP and dynactin were significantly reduced. Furthermore, the dynactin-P50 and βAPP that was present was located primarily in dystrophic neurites in Aβ plaques. Tissues from Alzheimer patients with APOE ε3,3 had less P-tau, more βAPP, dynactin-P50, and synaptophysin than did tissues from Alzheimer patients carrying APOE ε4,4. It is logical to conclude, then, that as neurons age successfully, there is coordination between retrograde delivery and maintenance and repair, as well as between retrograde delivery and degradation and/or recycling of spent proteins. The buildup of proteins slated for repair, synaptic viability, transport, and re-cycling in neuron soma and dystrophic neurites suggest a loss of this coordination in Alzheimer neurons. Inheritance of APOE ε3,3 rather than APOE ε4,4, is associated with neuronal resilience, suggestive of better repair capabilities, more synapses, more efficient transport, and less hyperphosphorylation of tau. We conclude that even in disease the ε3 allele is neuroprotective.
Keywords: APOE genotype; Alzheimer; P-tau; aging; dynactin-P50; motor proteins; synaptophysin; βAPP.
Figures

References
-
- Armstrong R. A., Myers D., Smith C. U. (1993). The spatial patterns of plaques and tangles in Alzheimer's disease do not support the ‘cascade hypothesis’. Dementia 4, 16–20. - PubMed
-
- Barger S. W., DeWall K. M., Liu L., Mrak R. E., Griffin W. S. (2008). Relationships between expression of apolipoprotein E and beta-amyloid precursor protein are altered in proximity to Alzheimer beta-amyloid plaques: potential explanations from cell culture studies. J. Neuropathol. Exp. Neurol. 67, 773–783. 10.1097/NEN.0b013e318180ec47 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

