Real-Time Monitoring of ATP-Responsive Drug Release Using Mesoporous-Silica-Coated Multicolor Upconversion Nanoparticles
- PMID: 25859611
- PMCID: PMC5808884
- DOI: 10.1021/acsnano.5b00641
Real-Time Monitoring of ATP-Responsive Drug Release Using Mesoporous-Silica-Coated Multicolor Upconversion Nanoparticles
Abstract
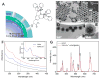
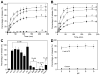
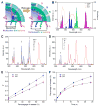
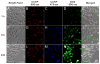
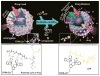
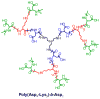
Stimuli-responsive drug delivery vehicles have garnered immense interest in recent years due to unparalleled progress made in material science and nanomedicine. However, the development of stimuli-responsive devices with integrated real-time monitoring capabilities is still in its nascent stage because of the limitations of imaging modalities. In this paper, we describe the development of a polypeptide-wrapped mesoporous-silica-coated multicolor upconversion nanoparticle (UCNP@MSN) as an adenosine triphosphate (ATP)-responsive drug delivery system (DDS) for long-term tracking and real-time monitoring of drug release. Our UCNP@MSN with multiple emission peaks in UV-NIR wavelength range was functionalized with zinc-dipicolylamine analogue (TDPA-Zn(2+)) on its exterior surface and loaded with small-molecule drugs like chemotherapeutics in interior mesopores. The drugs remained entrapped within the UCNP-MSNs when the nanoparticles were wrapped with a compact branched polypeptide, poly(Asp-Lys)-b-Asp, because of multivalent interactions between Asp moieties present in the polypeptide and the TDPA-Zn(2+) complex present on the surface of UCNP-MSNs. This led to luminescence resonance energy transfer (LRET) from the UCNPs to the entrapped drugs, which typically have absorption in UV-visible range, ultimately resulting in quenching of UCNP emission in UV-visible range while retaining their strong NIR emission. Addition of ATP led to a competitive displacement of the surface bound polypeptide by ATP due to its higher affinity to TDPA-Zn(2+), which led to the release of the entrapped drugs and subsequent elimination of LRET. Monitoring of such ATP-triggered ratiometric changes in LRET allowed us to monitor the release of the entrapped drugs in real-time. Given these results, we envision that our proposed UCNP@MSN-polypeptide hybrid nanoparticle has great potential for stimuli-responsive drug delivery as well as for monitoring biochemical changes taking place in live cancer and stem cells.
Keywords: core−shell nanoparticles; luminescence resonance energy transfer (LRET); real-time monitoring; stimuli-responsive drug delivery; upconversion nanoparticle.
Conflict of interest statement
Figures
Similar articles
-
Real-time monitoring of pH-responsive drug release using a metal-phenolic network-functionalized upconversion nanoconstruct.Nanoscale. 2019 May 9;11(18):9201-9206. doi: 10.1039/c9nr01892a. Nanoscale. 2019. PMID: 31038497
-
Amine-functionalized, porous silica-coated NaYF4:Yb/Er upconversion nanophosphors for efficient delivery of doxorubicin and curcumin.Mater Sci Eng C Mater Biol Appl. 2019 Mar;96:86-95. doi: 10.1016/j.msec.2018.11.007. Epub 2018 Nov 6. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30606601
-
Ratiometric Monitoring of Intracellular Drug Release by an Upconversion Drug Delivery Nanosystem.ACS Appl Mater Interfaces. 2015 Jun 10;7(22):12278-86. doi: 10.1021/acsami.5b03204. Epub 2015 May 26. ACS Appl Mater Interfaces. 2015. PMID: 25975535
-
Recent advances in mesoporous silica nanoparticles for antitumor therapy: our contribution.Biomater Sci. 2016 May 26;4(5):803-13. doi: 10.1039/c6bm00039h. Epub 2016 Feb 23. Biomater Sci. 2016. PMID: 26902682 Review.
-
Polymer-Brush-Grafted Mesoporous Silica Nanoparticles for Triggered Drug Delivery.Chemphyschem. 2018 Aug 17;19(16):1956-1964. doi: 10.1002/cphc.201800018. Epub 2018 Apr 14. Chemphyschem. 2018. PMID: 29575338 Review.
Cited by
-
Enrichment and sensing tumor cells by embedded immunomodulatory DNA hydrogel to inhibit postoperative tumor recurrence.Nat Commun. 2023 Jul 27;14(1):4511. doi: 10.1038/s41467-023-40085-4. Nat Commun. 2023. PMID: 37500633 Free PMC article.
-
Recent Advances on Inorganic Nanoparticle-Based Cancer Therapeutic Agents.Int J Environ Res Public Health. 2016 Nov 25;13(12):1182. doi: 10.3390/ijerph13121182. Int J Environ Res Public Health. 2016. PMID: 27898016 Free PMC article. Review.
-
Hurdles in selection process of nanodelivery systems for multidrug-resistant cancer.J Cancer Res Clin Oncol. 2016 Oct;142(10):2073-106. doi: 10.1007/s00432-016-2167-7. Epub 2016 Apr 26. J Cancer Res Clin Oncol. 2016. PMID: 27116692 Free PMC article. Review.
-
Mesoporous Silica Nanoparticles: Types, Synthesis, Role in the Treatment of Alzheimer's Disease, and Other Applications.Pharmaceutics. 2023 Nov 24;15(12):2666. doi: 10.3390/pharmaceutics15122666. Pharmaceutics. 2023. PMID: 38140007 Free PMC article. Review.
-
Theragnostic potentials of core/shell mesoporous silica nanostructures.Nanotheranostics. 2019 Jan 1;3(1):1-40. doi: 10.7150/ntno.27877. eCollection 2019. Nanotheranostics. 2019. PMID: 30662821 Free PMC article. Review.
References
-
- Mura S, Nicolas J, Couvreur P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat Mater. 2013;12:991–1003. - PubMed
-
- Alarcon CDH, Pennadam S, Alexander C. Stimuli Responsive Polymers for Biomedical Applications. Chem Soc Rev. 2005;34:276–285. - PubMed
-
- Bansal A, Zhang Y. Photocontrolled Nanoparticle Delivery Systems for Biomedical Applications. Acc Chem Res. 2014;47:3052–3060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

