Bioluminescence resonance energy transfer to detect protein-protein interactions in live cells
- PMID: 25859969
- PMCID: PMC4682348
- DOI: 10.1007/978-1-4939-2425-7_30
Bioluminescence resonance energy transfer to detect protein-protein interactions in live cells
Abstract
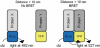
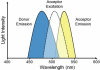
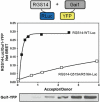
Bioluminescence resonance energy transfer (BRET) is a valuable tool to detect protein-protein interactions. BRET utilizes bioluminescent and fluorescent protein tags with compatible emission and excitation properties, making it possible to examine resonance energy transfer when the tags are in close proximity (<10 nm) as a typical result of protein-protein interactions. Here we describe a protocol for detecting BRET from two known protein binding partners (Gαi1 and RGS14) in HEK 293 cells using Renilla luciferase and yellow fluorescent protein tags. We discuss the calculation of the acceptor/donor ratio as well as net BRET and demonstrate that BRET can be used as a platform to investigate the regulation of protein-protein interactions in live cells in real time.
Figures
References
-
- Wu P, Brand L. Resonance energy transfer: methods and applications. Anal Biochem. 1994;218:1–13. - PubMed
-
- Xu Y, Kanauchi A, von Arnim AG, et al. Bioluminescence resonance energy transfer: monitoring protein-protein interactions in living cells. Methods Enzymol. 2003;360:289–301. - PubMed
-
- Lohse MJ, Nuber S, Hoffmann C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol Rev. 2012;64:299–336. - PubMed
-
- Nagai T, Ibata K, Park ES, et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

