Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig
- PMID: 25860138
- PMCID: PMC4393265
- DOI: 10.1371/journal.pone.0122198
Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig
Abstract
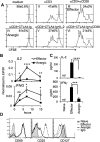
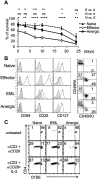
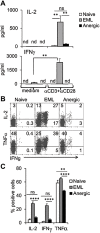
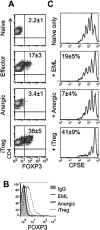
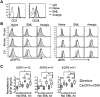
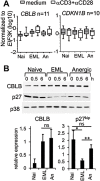
During activation, T cells integrate multiple signals from APCs and cytokine milieu. The blockade of these signals can have clinical benefits as exemplified by CTLA4-Ig, which blocks interaction of B7 co-stimulatory molecules on APCs with CD28 on T cells. Variants of CTLA4-Ig, abatacept and belatacept are FDA approved as immunosuppressive agents in arthritis and transplantation, yet murine studies suggested that CTLA4-Ig could be beneficial in a number of other diseases. However, detailed analysis of human CD4 cell hyporesponsivness induced by CTLA4-Ig has not been performed. Herein, we established a model to study the effect of CTLA4-Ig on the activation of human naïve T cells in a human mixed lymphocytes system. Comparison of human CD4 cells activated in the presence or absence of CTLA4-Ig showed that co-stimulation blockade during TCR activation does not affect NFAT signaling but results in decreased activation of NF-κB and AP-1 transcription factors followed by a profound decrease in proliferation and cytokine production. The resulting T cells become hyporesponsive to secondary activation and, although capable of receiving TCR signals, fail to proliferate or produce cytokines, demonstrating properties of anergic cells. However, unlike some models of T cell anergy, these cells did not possess increased levels of the TCR signaling inhibitor CBLB. Rather, the CTLA4-Ig-induced hyporesponsiveness was associated with an elevated level of p27kip1 cyclin-dependent kinase inhibitor.
Conflict of interest statement
Figures


Similar articles
-
T cell effector function and anergy avoidance are quantitatively linked to cell division.J Immunol. 2000 Sep 1;165(5):2432-43. doi: 10.4049/jimmunol.165.5.2432. J Immunol. 2000. PMID: 10946268
-
Evidence that induction of tolerance in vivo involves active signaling via a B7 ligand-dependent mechanism: CTLA4-Ig protects V beta 8+ T cells from tolerance induction by the superantigen staphylococcal enterotoxin B.Eur J Immunol. 1996 Apr;26(4):858-62. doi: 10.1002/eji.1830260420. Eur J Immunol. 1996. PMID: 8625979
-
CTLA4 blockade maximizes antitumor T-cell activation by dendritic cells presenting idiotype protein or opsonized anti-CD20 antibody-coated lymphoma cells.J Immunother. 2002 Nov-Dec;25(6):455-68. doi: 10.1097/00002371-200211000-00002. J Immunother. 2002. PMID: 12439343
-
Co-stimulatory modulation in rheumatoid arthritis: the role of (CTLA4-Ig) abatacept.Autoimmun Rev. 2008 Oct;8(1):76-82. doi: 10.1016/j.autrev.2008.07.035. Epub 2008 Aug 15. Autoimmun Rev. 2008. PMID: 18718877 Review.
-
The role of co-stimulation in airway inflammation.Clin Exp Allergy. 2000 Jun;30 Suppl 1:46-50. doi: 10.1046/j.1365-2222.2000.00097.x. Clin Exp Allergy. 2000. PMID: 10849475 Review.
Cited by
-
BioWardrobe: an integrated platform for analysis of epigenomics and transcriptomics data.Genome Biol. 2015 Aug 7;16(1):158. doi: 10.1186/s13059-015-0720-3. Genome Biol. 2015. PMID: 26248465 Free PMC article.
-
CD28 Costimulation: From Mechanism to Therapy.Immunity. 2016 May 17;44(5):973-88. doi: 10.1016/j.immuni.2016.04.020. Immunity. 2016. PMID: 27192564 Free PMC article. Review.
-
CTLA4-Ig treatment induces M1-M2 shift in cultured monocyte-derived macrophages from healthy subjects and rheumatoid arthritis patients.Arthritis Res Ther. 2021 Dec 24;23(1):306. doi: 10.1186/s13075-021-02691-9. Arthritis Res Ther. 2021. PMID: 34952630 Free PMC article.
-
Co-stimulatory and co-inhibitory pathways in cancer immunotherapy.Adv Cancer Res. 2019;143:145-194. doi: 10.1016/bs.acr.2019.03.003. Epub 2019 Apr 17. Adv Cancer Res. 2019. PMID: 31202358 Free PMC article. Review.
-
Reversible suppression of T cell function in the bone marrow microenvironment of acute myeloid leukemia.Proc Natl Acad Sci U S A. 2020 Jun 23;117(25):14331-14341. doi: 10.1073/pnas.1916206117. Epub 2020 Jun 8. Proc Natl Acad Sci U S A. 2020. PMID: 32513686 Free PMC article.
References
-
- Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M (2002) Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol 14: 592–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

