Comparison of UHPLC and HPLC in benzodiazepines analysis of postmortem samples: a case-control study
- PMID: 25860209
- PMCID: PMC4554044
- DOI: 10.1097/MD.0000000000000640
Comparison of UHPLC and HPLC in benzodiazepines analysis of postmortem samples: a case-control study
Abstract
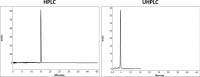
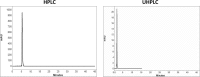
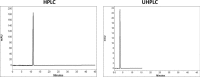
The aim of this study was to compare system efficiency and analysis duration regarding the solvent consumption and system maintenance in high-pressure liquid chromatography (HPLC) and ultra high-pressure liquid chromatography (UHPLC). In a case-control study, standard solutions of 7 benzodiazepines (BZs) and 73 biological samples such as urine, tissue, stomach content, and bile that screened positive for BZs were analyzed by HPLC and UHPLC in laboratory of forensic toxicology during 2012 to 2013. HPLC analysis was performed using a Knauer by 100-5 C-18 column (250 mm × 4.6 mm) and Knauer photodiode array detector (PAD). UHPLC analysis was performed using Knauer PAD detector with cooling autosampler and Eurospher II 100-3 C-18 column (100 mm × 3 mm) and also 2 pumps. The mean retention time, standard deviation, flow rate, and repeatability of analytical results were compared by using 2 methods. Routine runtimes in HPLC and UHPLC took 40 and 15 minutes, respectively. Changes in mobile phase composition of the 2 methods were not required. Flow rate and solvent consumption in UHPLC decreased. Diazepam and flurazepam were detected more frequently in biological samples. In UHPLC, small particle size and short length of column cause effective separation of BZs in a very short time. Reduced flow rate, solvent consumption, and injection volume cause more efficiency and less analysis costs. Thus, in the detection of BZs, UHPLC is an accurate, sensitive, and fast method with less cost of analysis.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
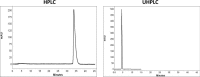
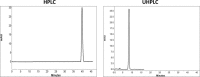
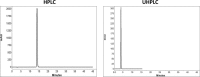
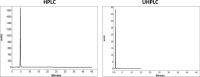
Similar articles
-
High perfomance liquid chromatography in pharmaceutical analyses.Bosn J Basic Med Sci. 2004 May;4(2):5-9. doi: 10.17305/bjbms.2004.3405. Bosn J Basic Med Sci. 2004. PMID: 15629016 Free PMC article.
-
Determination of 12 commonly found compounds in DUID cases in whole blood using fully automated supported liquid extraction and UHPLC-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Sep 1;1093-1094:8-23. doi: 10.1016/j.jchromb.2018.06.050. Epub 2018 Jun 25. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 29980102
-
Screening, identification, and quantitation of benzodiazepines in postmortem samples by HPLC with photodiode array detection.J Anal Toxicol. 1995 Jan-Feb;19(1):35-40. doi: 10.1093/jat/19.1.35. J Anal Toxicol. 1995. PMID: 7723300
-
A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010-2019).Phytochem Anal. 2020 Jul;31(4):413-457. doi: 10.1002/pca.2906. Epub 2019 Dec 17. Phytochem Anal. 2020. PMID: 31849137 Review.
-
Methods for the measurement of benzodiazepines in biological samples.J Chromatogr B Biomed Sci Appl. 1998 Aug 21;713(1):201-25. doi: 10.1016/s0378-4347(97)00537-9. J Chromatogr B Biomed Sci Appl. 1998. PMID: 9700560 Review.
Cited by
-
Challenges and future directions in LC-MS-based multiclass method development for the quantification of food contaminants.Anal Bioanal Chem. 2021 Jan;413(1):25-34. doi: 10.1007/s00216-020-03015-7. Epub 2020 Nov 13. Anal Bioanal Chem. 2021. PMID: 33188454 Free PMC article.
-
Benzodiazepines in complex biological matrices: Recent updates on pretreatment and detection methods.J Pharm Anal. 2023 May;13(5):442-462. doi: 10.1016/j.jpha.2023.03.007. Epub 2023 Apr 1. J Pharm Anal. 2023. PMID: 37305786 Free PMC article. Review.
-
Can precision antibiotic prescribing help prevent the spread of carbapenem-resistant organisms in the hospital setting?JAC Antimicrob Resist. 2023 Mar 29;5(2):dlad036. doi: 10.1093/jacamr/dlad036. eCollection 2023 Apr. JAC Antimicrob Resist. 2023. PMID: 37008824 Free PMC article. Review.
-
The Development of a Rapid, Cost-Effective, and Green Analytical Method for Mercury Speciation.Toxics. 2024 Jun 11;12(6):424. doi: 10.3390/toxics12060424. Toxics. 2024. PMID: 38922104 Free PMC article.
-
The hidden treasures in endophytic fungi: a comprehensive review on the diversity of fungal bioactive metabolites, usual analytical methodologies, and applications.Arch Microbiol. 2024 Mar 20;206(4):185. doi: 10.1007/s00203-024-03911-x. Arch Microbiol. 2024. PMID: 38506928 Review.
References
-
- Brunton L, Lazo JS, Parker K. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th1996; New York: McGraw-Hill Companies, Inc., 6–19.
-
- Drummer OH. Benzodiazepines-effects on human performance and behavior. Forensic Sci Rev 2002; 14:1–2. - PubMed
-
- Kratzsch C, Tenberken O, Peters FT, et al. Screening library-assisted identification and validated quantification of 23 benzodiazepines, flumazenil, zaleplon and zolpidem in plasma by liquid chromatography/mass spectrometry with atmospheric pressure chemical ionization. J Mass Spectrom 2004; 39:656–872. - PubMed
-
- Schlueter S, Hutchison J. Identification and Quantitation of BZ and Metabolites by LC/MS Clinical and Forensic Toxicology Agilent Technology. 2006;34:5–14.
-
- Dempsey Wu N, Yehl J, Dovletoglou PM, et al. Practical aspects of fast HPLC separations for pharmaceutical process development using Monolithic Columns. Anal Chim Acta 2004; 523:149–156.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

