Roles of GasderminA3 in Catagen-Telogen Transition During Hair Cycling
- PMID: 25860385
- PMCID: PMC4537385
- DOI: 10.1038/jid.2015.147
Roles of GasderminA3 in Catagen-Telogen Transition During Hair Cycling
Abstract
Hair follicles undergo cyclic behavior through regression (catagen), rest (telogen), and regeneration (anagen) during postnatal life. The hair cycle transition is strictly regulated by the autonomous and extrinsic molecular environment. However, whether there is a switch controlling catagen-telogen transition remains largely unknown. Here we show that hair follicles cycle from catagen to the next anagen without transitioning through a morphologically typical telogen after Gsdma3 mutation. This leaves an ESLS (epithelial strand-like structure) during the time period corresponding to telogen phase in WT mice. Molecularly, Wnt10b is upregulated in Gsdma3 mutant mice. Restoration of Gsdma3 expression in AE (alopecia and excoriation) mouse skin rescues hair follicle telogen entry and significantly decreases the Wnt10b-mediated Wnt/β-catenin signaling pathway. Overexpression of Wnt10b inhibits telogen entry by increasing epithelial strand cell proliferation. Subsequently, hair follicles with a Gsdma3 mutation enter the second anagen simultaneously as WT mice. Hair follicles cannot enter the second anagen with ectopic WT Gsdma3 overexpression. A luciferase reporter assay proves that Gsdma3 directly suppresses Wnt signaling. Our findings suggest that Gsdma3 has an important role in catagen-telogen transition by balancing the Wnt signaling pathway and that morphologically typical telogen is not essential for the initiation of a new hair cycle.
Conflict of interest statement
The authors state no conflict of interest.
Figures

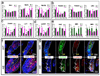
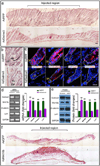
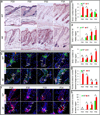
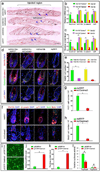

References
-
- Andl T, Ahn K, Kairo A, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
-
- Foitzik K, Lindner G, Mueller-Roever S, et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000;14:752–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

