Ubiquitous L1 mosaicism in hippocampal neurons
- PMID: 25860606
- PMCID: PMC4398972
- DOI: 10.1016/j.cell.2015.03.026
Ubiquitous L1 mosaicism in hippocampal neurons
Abstract
Somatic LINE-1 (L1) retrotransposition during neurogenesis is a potential source of genotypic variation among neurons. As a neurogenic niche, the hippocampus supports pronounced L1 activity. However, the basal parameters and biological impact of L1-driven mosaicism remain unclear. Here, we performed single-cell retrotransposon capture sequencing (RC-seq) on individual human hippocampal neurons and glia, as well as cortical neurons. An estimated 13.7 somatic L1 insertions occurred per hippocampal neuron and carried the sequence hallmarks of target-primed reverse transcription. Notably, hippocampal neuron L1 insertions were specifically enriched in transcribed neuronal stem cell enhancers and hippocampus genes, increasing their probability of functional relevance. In addition, bias against intronic L1 insertions sense oriented relative to their host gene was observed, perhaps indicating moderate selection against this configuration in vivo. These experiments demonstrate pervasive L1 mosaicism at genomic loci expressed in hippocampal neurons.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

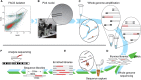



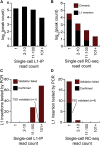
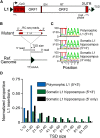

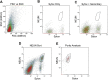

References
-
- Boissinot S., Entezam A., Furano A.V. Selection against deleterious LINE-1-containing loci in the human lineage. Mol. Biol. Evol. 2001;18:926–935. - PubMed
Supplemental References
-
- Feng Q., Moran J.V., Kazazian H.H., Jr., Boeke J.D. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell. 1996;87:905–916. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

