Melanocortins contribute to sequential differentiation and enucleation of human erythroblasts via melanocortin receptors 1, 2 and 5
- PMID: 25860801
- PMCID: PMC4393082
- DOI: 10.1371/journal.pone.0123232
Melanocortins contribute to sequential differentiation and enucleation of human erythroblasts via melanocortin receptors 1, 2 and 5
Abstract
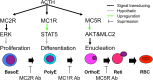
In this study, we showed that adrenocorticotropic hormone (ACTH) promoted erythroblast differentiation and increased the enucleation ratio of erythroblasts. Because ACTH was contained in hematopoietic medium as contamination, the ratio decreased by the addition of anti-ACTH antibody (Ab). Addition of neutralizing Abs (nAbs) for melanocortin receptors (MCRs) caused erythroblast accumulation at specific stages, i.e., the addition of anti-MC2R nAb led to erythroblast accumulation at the basophilic stage (baso-E), the addition of anti-MC1R nAb caused accumulation at the polychromatic stage (poly-E), and the addition of anti-MC5R nAb caused accumulation at the orthochromatic stage (ortho-E). During erythroblast differentiation, ERK, STAT5, and AKT were consecutively phosphorylated by erythropoietin (EPO). ERK, STAT5, and AKT phosphorylation was inhibited by blocking MC2R, MC1R, and MC5R, respectively. Finally, the phosphorylation of myosin light chain 2, which is essential for the formation of contractile actomyosin rings, was inhibited by anti-MC5R nAb. Taken together, our study suggests that MC2R and MC1R signals are consecutively required for the regulation of EPO signal transduction in erythroblast differentiation, and that MC5R signal transduction is required to induce enucleation. Thus, melanocortin induces proliferation and differentiation at baso-E, and polarization and formation of an actomyosin contractile ring at ortho-E are required for enucleation.
Conflict of interest statement
Figures

Similar articles
-
Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved.Mol Cell Endocrinol. 2011 Jul 20;341(1-2):9-17. doi: 10.1016/j.mce.2011.03.010. Epub 2011 May 17. Mol Cell Endocrinol. 2011. PMID: 21616121
-
Melanocortins induce interleukin 6 gene expression and secretion through melanocortin receptors 2 and 5 in 3T3-L1 adipocytes.J Mol Endocrinol. 2010 Apr;44(4):225-36. doi: 10.1677/JME-09-0161. Epub 2010 Jan 20. J Mol Endocrinol. 2010. PMID: 20089716 Free PMC article.
-
Development of potent selective competitive-antagonists of the melanocortin type 2 receptor.Mol Cell Endocrinol. 2014 Aug 25;394(1-2):99-104. doi: 10.1016/j.mce.2014.07.003. Epub 2014 Jul 11. Mol Cell Endocrinol. 2014. PMID: 25017734
-
60 YEARS OF POMC: Melanocortin receptors: evolution of ligand selectivity for melanocortin peptides.J Mol Endocrinol. 2016 May;56(4):T119-33. doi: 10.1530/JME-15-0292. Epub 2016 Jan 20. J Mol Endocrinol. 2016. PMID: 26792827 Review.
-
Using the human melanocortin-2 receptor as a model for analyzing hormone/receptor interactions between a mammalian MC2 receptor and ACTH(1-24).Gen Comp Endocrinol. 2013 Jan 15;181:203-10. doi: 10.1016/j.ygcen.2012.11.011. Epub 2012 Nov 29. Gen Comp Endocrinol. 2013. PMID: 23201148 Review.
Cited by
-
Atypical pituitary hormone-target tissue axis.Front Med. 2023 Feb;17(1):1-17. doi: 10.1007/s11684-022-0973-7. Epub 2023 Feb 27. Front Med. 2023. PMID: 36849623 Review.
-
Immunoregulatory properties of erythroid nucleated cells induced from CD34+ progenitors from bone marrow.PLoS One. 2023 Jun 30;18(6):e0287793. doi: 10.1371/journal.pone.0287793. eCollection 2023. PLoS One. 2023. PMID: 37390055 Free PMC article.
-
Comprehensive genetic testing combined with citizen science reveals a recently characterized ancient MC1R mutation associated with partial recessive red phenotypes in dog.Canine Med Genet. 2020 Nov 5;7(1):16. doi: 10.1186/s40575-020-00095-7. Canine Med Genet. 2020. PMID: 33292722 Free PMC article.
-
Industrially Compatible Transfusable iPSC-Derived RBCs: Progress, Challenges and Prospective Solutions.Int J Mol Sci. 2021 Sep 10;22(18):9808. doi: 10.3390/ijms22189808. Int J Mol Sci. 2021. PMID: 34575977 Free PMC article. Review.
-
Leukemia Inhibitory Factor Induces Proopiomelanocortin via CRH/CRHR Pathway in Mouse Trophoblast.Front Cell Dev Biol. 2021 Jul 19;9:618947. doi: 10.3389/fcell.2021.618947. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34350170 Free PMC article.
References
-
- Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005; 23: 69–74. - PubMed
-
- Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006; 24: 1255–1256. - PubMed
-
- McNiece IK, Langley KE, Zsebo KM. Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol. 1991; 19: 226–231. - PubMed
-
- Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarrantana MC, et al. (2002) Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002; 20: 467–472. - PubMed
-
- Chen C, Sytkowski AJ. Erythropoietin regulation of Raf-1 and MEK: evidence for a Ras-independent mechanism. Blood. 2004; 104: 73–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

